Hemp and linum disease models
Sclerotinia rot
Sclerotinia rot affects a wide range of plants particularly non-woody species. Sclerotinia rot is caused by S. sclerotiorum. Sclerotinia rot can affect plants at any stage of production including seedlings, mature plants and harvested products. Plants with senescing or dead tissue are particularly susceptible to infection.
Symptoms
The infected area of a plant initially takes on a dark green or brown water-soaked appearance, then may become paler in color. Dense white cottony mycelium usually develops and the plant begins to wilt and eventually dies. Resting or survival structures (sclerotia) are produced externally on affected plant parts and internally in stem pith cavities. The sclerotia are hard, black, irregular in shape, mostly 2-4 mm in size, and difficult to see once incorporated into the soil.
Disease sources and spread
The life-cycle of S. sclerotiorum includes both a soil-borne and an air-borne phase. Sclerotia of S. sclerotiorum can survive in the soil for ten years or more. They germinate to produce small funnel-shaped fruiting bodies (apothecia) that are approximately 1 cm in diameter. Apothecia produce air-borne spores, which can cause infection when they land on a susceptible host plant, either via flowers, or by direct germination on leaves. Occasionally, infection of stem bases can occur when fungal strands (mycelium) develop directly from Sclerotia near the surface. New sclerotia develop in infected plant tissue and when the plant dies they remain on the soil surface or may become incorporated during subsequent soil cultivation.
Conditions for Infection
After a period of cold conditions in winter, sclerotia, overwintering in the top 5 cm of the soil, germinate from spring onwards to produce apothecia, when soil temperatures are 10°C or higher and the soil is moist. Sclerotia do not germinate in dry soil or when the soil temperature is above 25°C. Sclerotia buried below 5 cm in the soil are less likely to germinate. Once apothecia are fully formed, spore release can occur in the light or dark but is temperature dependent, so tends to peak around midday. Apothecia can last about 20 days at 15 to 20°C, but shrivel after less than 10 days at 25°C. For flowering herbs, spores landing on petals and stamens germinate rapidly (germination within 3-6 hours and infection within 24 hours) in optimum conditions of 15-25°C, continuous leaf wetness and high humidity within the crop. Subsequent infection of leaves and stems depends on petals falling and sticking on leaves. The risk of infection is increased if the leaves are wet because this causes more petals to stick. Infected dead or senescing petals provide nutrients for the invasion of the fungus into leaves and stems. For non-flowering herbs, infection is mainly by air-borne spores landing directly on leaves. Spores can survive on leaves for several weeks until conditions favorable for leaf infection occur. Spore germination and infection depend on the presence of nutrients on leaves, either from plant wounds or senescing plant material. As for flowering herbs, the optimum spore germination and infection conditions are 15-25°C with continuous leaf wetness and high humidity. Once plant infection has occurred, rapid disease progress is favored by warm (15-20°C) and moist conditions in dense crops.
Sclerotinia Infection Model
Plant Infection by S. sclerotiorum
Carpogenic germination of sclerotia is stimulated by periods of continuous soil moisture. Apothecia are formed on the soil surface from which ascospores are released into the air. Infection of most crop species is principally associated with ascospores but direct infection of healthy, intact plant tissue from germinating ascospores usually does not occur. Instead, infection of leaf and stem tissue of healthy plants results only when germinating ascospores colonize dead or senescing tissues, usually flower parts such as abscised petals, prior to the formation of infection structures and penetration. Myceliogenic germination of sclerotia at the soil surface can also result in colonization of dead organic matter with subsequent infection of adjacent living plants. However, in some crops, for example sunflower myceliogenic germination of sclerotia can directly initiate the infection process of the roots and basal stem resulting in wilt. The stimulus for myceliogenic germination and infection in sunflower is not known but likely depends on nutritional signals in the rhizosphere derived from host plants.
The infection process
Infection of healthy tissue depends on the formation of an appressorium, which may be simple or complex in structure depending on the host surface. In most cases, penetration is directly through the cuticle and not through stomata. Appressoria develop from terminal dichotomous branching of hyphae growing on the host surface and consist of a pad of broad, multi-septate, short hyphae that are orientated perpendicular to the host surface to which they are attached by mucilage. Complex appressoria are often referred to as infection cushions. Although earlier workers considered penetration of the cuticle to be a purely mechanical process there is strong evidence from ultra-structural studies that enzymatic digestion of the cuticle also plays a role in the penetration process. Little is known about S. sclerotiorum cutinases, however, the genome encodes at least four cutinase-like enzymes (Hegedus unpublished). A large vesicle, formed at the appressorium tip prior to penetration, appears to be released into the host cuticle during penetration. After penetration of the cuticle, a subcuticular vesicle forms from which large hyphae fan out growing over and dissolving the subcuticular wall of the epidermis.
Infection by enzymatic degratation of the epidemic cells: Oxalic acid works in concern with cell wall degrading enzymes, such as polygalacturonase (PG), to bring about the destruction of host tissue by creating an environment conducive for PG attack on pectin in the middle lamella. This in turn releases low molecular weight derivatives that induce the expression of additional PG genes. Indeed, overall PG activity is induced by pectin or pectin-derived monosaccharides, such as galacturonic acid, and is repressed by the presence of glucose. Examination of the expression patterns of individual Sspg genes has revealed that the interplay among PGs and with the host during the various stages of infection is finely co-ordinated. (Dwayne D. Hegedus *, S. Roger Rimmer: Sclerotinia sclerotiorum: When ‘‘to be or not to be’’ a pathogen? FEMS Microbiology Letters 251 (2005) 177–184)
Looking for Climate Conditions for Infection of S. sclerotiorum has to take consideration of the apothecia formation, the sporulation, the direct infection by apothecia (even if it does not take place very frequent) and the infection from established mycelia by encymatic degradation of the epidemic cells .
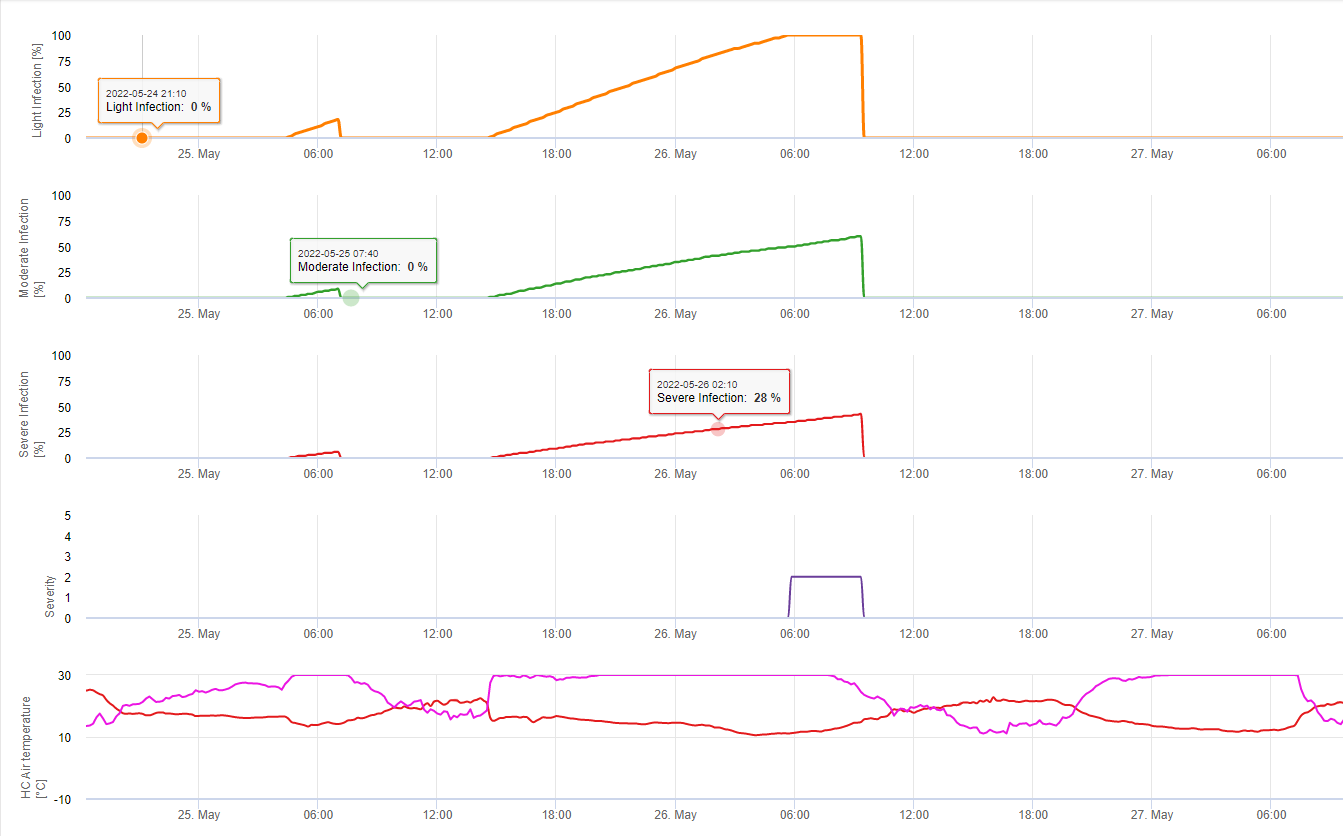
Apothecia formation and sporulation takes place if a rain of more than 8 mm is followed by a period of high relative humdiity lasting longer than 20 hours at optimum temperature of 21°C to 26°C.
Direct Infection by Apothecia can be expected after a leaf wetness period followed by 16 hours of relative humdity higher than 90% under optimum 21°C to 26°C (“appressoria infection”). Wheras saprophytic growth followed by encymatic degratation of the epidermic cells (“hydrolytic infection”) can be expected under a slightly lower relative humditiy of 80% lasting for a period of 24 hours under optimum conditions of 21°C to 26°C.
Literature:
- Lumsden, R.D. (1976) Pectolytic enzymes of Sclerotinia sclerotiorum and their localization on infected bean. Can. J. Bot. 54,2630–2641.
Tariq, V.N. and Jeffries, P. (1984) Appressorium formation by Sclerotinia sclerotiorum: scanning electron microscopy. Trans. Brit. Mycol. Soc. 82, 645–651. - Boyle, C. (1921) Studies in the physiology of parasitism. VI. Infection by Sclerotinia libertiana. Ann. Bot. 35, 337–347.
Abawi, G.S., Polach, F.J. and Molin, W.T. (1975) Infection of bean by ascospores of Whetzelinia sclerotiorum. Phytopathology 65, 673–678. - Tariq, V.N. and Jeffries, P. (1986) Ultrastructure of penetration of Phaseolus spp. by Sclerotinia sclerotiorum. Can. J. Bot. 64, 2909– 2915.
- Marciano, P., Di Lenna, P. and Magro, P. (1983) Oxalic acid, cell wall degrading enzymes and pH in pathogenesis and their significance in the virulence of two Sclerotinia sclerotiorum isolates on sunflower. Physiol. Plant Pathol. 22, 339–345.
- Fraissinet-Tachet, L. and Fevre, M. (1996) Regulation by galacturonic acid of ppectinolytic enzyme production by Sclerotinia sclerotiorum. Curr. Microbiol. 33, 49–53.
Practical Use of the Sclerotinia Model
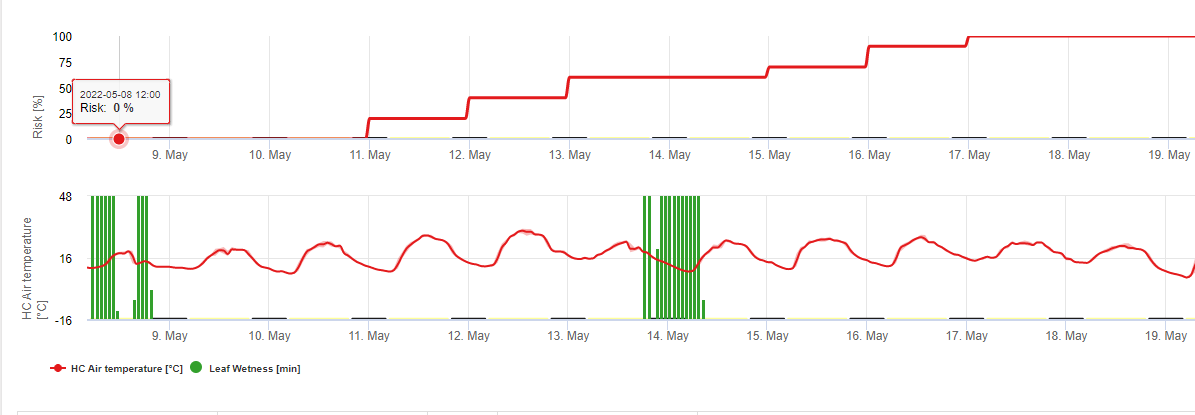
The White Leg Infection Model shows the periods when the formation of apothecia are expected. If these periods are coinsitent with the flowering period of rape seed or canola we have to expect S. sclerotiorum infections during a moist period. The spores formed in the apothecia might be available for one to several days. The opportunity of infections is indicated by the calculation of the infection progress for direct or indirect infections by appressoria or encymatic cell wall degratation. If the infection progress line reaches 100% an infection has to be assumed. These infections should be covered preventative or a fungicid with a curative action against S. sclerotiorum has to be used.
Grey mould
Grey Mould Biology
Grey Mould (Botrytis cinerea) is a devastating disease with high economic impact in production. B. cinerea infects the flowers and the fruits close to maturity.
The fungal pathogen has a very broad host range, infecting more than 200 different hosts. Fungal growth exists saprophytically and parasitic.
Symptoms
On Sunflowers the pathogen causes a grey mould on the head and stem. Therewhile the leaves start to dry out. These symptoms occur during maturation of kernels on the head. Brown spots on the back side are seen. These spots are covered by the fungal mycelium and spores, giving the appearance of a powdery. Spores are able to be spread during wet weather conditions.
Black sclerotia deprived of medulla appear on the crop debris after harvesting or directly on the plants, if they are harvested too late.
The fungus overwinters during winter on the soil surface or in the soil as mycelium or sclerotia. In springtime the overwintering form starts to germinate and produce conidia. These conidia are spread by wind and rain and infect new plant tissue.
Germination is possible at relative humidity over 85%. The optimal germinating temperature is 18°C. The fungal pathogen can reproduce multiply times.
Control options: Seed control can protect plants of damping- off. Chemical control is difficult due to the resistance of the pathogen. Therefore attempts are made for natural control strategies with Trichoderma harzianum.
Conditions for modeling infection
B. cinerea infections are related to free moisture. Therefore in open field production leaf wetness, which is a good indicator, is determined.
Bulger et al. (1987) studied the correlation of leaf wetness periods during flowering and the occurrence of grey mould on the fruits. They found that for a higher risks of infection at 20°C a time periode of longer than 32 hours of leaf wetness is needed. At lower temperatures the leaf wetness periods have to be longer for infection of the disease.
FieldClimate is indicating risk of Botrytis cinerea on base of leaf wetness periods and the temperature during these periods.
The graph below shows the duration of wet leaves in dependence of the actual temperature needed for a Botrytis infection. If the risk is higher than 0 every leaf wetness period longer than 4 hours will increase the risk by the same relation.
A day with a leaf wetness period shorter than 4 hours is assumed to be a dry day and will reduce the risk by 20% of the actual value.
Practical use of the Grey Mould Model: The model indicates periods with a risk of a Botrytis infection. This risk period during the bloom of strawberry will lead to infected fruits. As longer the risk period lasts and as higher the risk is as higher is the probability and the number of infected fruit. The risk, which can be taken under consideration, depends on the market. Growers, which are selling their fruits to the supermarket will not take any risk, knowing that they are not able to sell infected fruits. While growers, who sell their fruits directly to the people are able to take a higher risk.
Literature:
- Bulger M.A., Ellis M. A., Madden L. V. (1987): Influence of temperature and wetness duration on infection of strawberry flowers by Botrytis cinerea and disease incidence of fruit originating from infected flowers. Ecology and Epidemiology; Vol 77 (8): 1225-1230.
- Sosa-Alvarez M., Madden L.V., Ellis M.A. (1995): Effects of temperature and wetness duration on sporulation of Botrytis cinerea on strawberry leaf residues. Plant disease 79, 609-615.
Brown blight
Early Blight of Potato and Tomato
Randall C. Rowe, Sally A. Miller, Richard M. Riedel, Ohio State University Extension Service
Early blight is a very common disease of both potato and tomato. It causes leaf spots and tuber blight on potato, and leaf spots, fruit rot and stem lesions on tomato. The disease can occur over a wide range of climatic conditions and can be very destructive if left uncontrolled, often resulting in complete defoliation of plants. In contrast to the name, it rarely develops early, but usually appears on mature foliage.
Symptoms
On leaves of both crops, the first symptoms usually appear on older leaves and consist of small, irregular, dark brown to black, dead spots ranging in size from a pinpoint to 1/2 inch in diameter. As the spots enlarge, concentric rings may form as a result of irregular growth patterns by the organism in the leaf tissue. This gives the lesion a characteristic “target-spot” or “bull’s eye” appearance. There is often a narrow, yellow halo around each spot and lesions are usually bordered by veins. When spots are numerous, they may grow together, causing infected leaves to turn yellow and die. Usually the oldest leaves become infected first and they dry up and drop from the plant as the disease progresses up the main stem.
On tomato, stem infections can occur at any age resulting in small, dark, slightly sunken areas that enlarge to form circular or elongated spots with lighter-coloured centers. Concentric markings, similar to those on leaves, often develop on stem lesions. If infested seed are used to start tomato transplants, seedlings may damp off soon after emergence. When large lesions develop at the ground line on stems of transplants or seedlings, the plants may become girdled, a condition known as “collar rot.” Such plants may die when set in the field or, if stems are weakened, may break over early in the season. Some plants may survive with reduced root systems if portions of stems above the canker develop roots where they contact the soil. Such plants, however, usually produce few or no fruits. Stem lesions are much less common and destructive on potato.
Blossom drop and spotting of fruit stems, along with loss of young fruit, may occur when early blight attacks tomatoes in the flowering stage. On older fruits, early blight causes dark, leathery sunken spots, usually at the point of stem attachment. These spots may enlarge to involve the entire upper portion of the fruit, often showing concentric markings like those on leaves. Affected areas may be covered with velvety black masses of spores. Fruits can also be infected in the green or ripe stage through growth cracks and other wounds. Infected fruits often drop before they reach maturity.
On potato tubers, early blight results in surface lesions that appear a little darker than adjacent healthy skin. Lesions are usually slightly sunken, circular or irregular, and vary in size up to 3/4 inch in diameter. There is usually a well defined and sometimes slightly raised margin between healthy and diseased tissue. Internally, the tissue shows a brown to black corky, dry rot, usually not more than 1/4 to 3/8 inch deep. Deep cracks may form in older lesions. Tuber infection is uncommon under Ohio conditions.
Pathogen
Early blight is caused by the fungus, Alternaria solani, which survives in infected leaf or stem tissues on or in the soil. This fungus is universally present in fields where these crops have been grown. It can also be carried on tomato seed and in potato tubers. Spores form on infested plant debris at the soil surface or on active lesions over a fairly wide temperature range, especially under alternating wet and dry conditions. They are easily carried by air currents, windblown soil, splashing rain, and irrigation water. Infection of susceptible leaf or stem tissues occurs in warm, humid weather with heavy dews or rain. Early blight can develop quite rapidly in mid to late season and is more severe when plants are stressed by poor nutrition, drought, or other pests. Infection of potato tubers occurs through natural openings on the skin or through injuries. Tubers may come in contact with spores during harvest and lesions may continue to develop in storage.
TOMCAST (Jim Jasinski, TOMCAST Coordinator FOR OHIO, INDIANA, & MICHIGAN)
Background
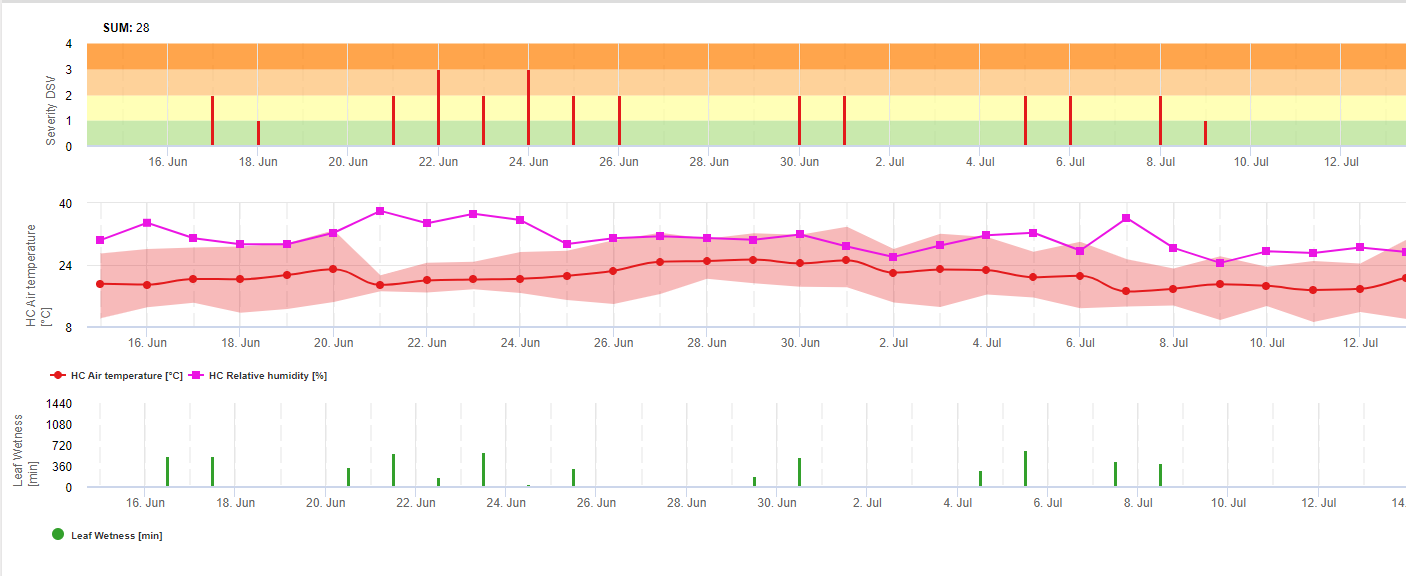
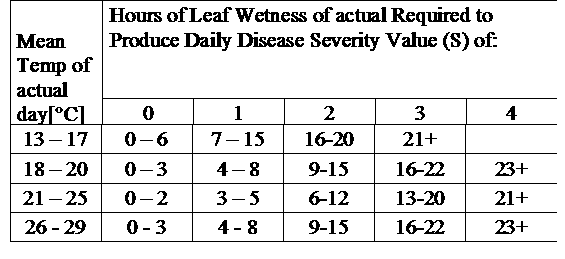
TOMCAST (TOMato disease foreCASTing) is a computer model based on field data that attempts to predict fungal disease development, namely Early Blight, Septoria Leaf Spot and Anthracnose on tomatoes. Field placed data loggers are recording hourly leaf wetness and temperature data. This data where analysed over a 24 hour period and may result in the formation of a Disease Severity Value (DSV); essentially an increment of disease development. As DSV accumulate, disease pressure continues to build on the crop. When the number of accumulated DSV exceed the spray interval, a fungicide application is recommended to relieve the disease pressure.
TOMCAST
Timing fungicide applications for early blight, Septoria leaf spot, and Anthracnose
A system of weather-based disease forecasting called TOMCAST, developed by Dr. Ron Pitblado at the Ridgetown College of Agricultural Technology in Ontario, Canada, can be used to time fungicide applications for three fungal diseases; early blight (caused by Alternaria solani), Septoria leaf spot (caused by Septoria lycopersici), and fruit anthracnose (caused by Colletotrichum coccodes). If late blight is present in your county or adjacent counties, or conditions are present for movement of spores into your area, use the Simcast late blight forecast system to time fungicide applications.
IMPORTANT CAUTIONARY NOTE:
TOMCAST is not useful on farms that have a history of bacterial diseases. If you commonly have problems with bacterial spot, speck, or canker in your tomatoes, you should not use TOMCAST because the recommended spray intervals will not be sufficient for control of bacterial diseases if you’re tank-mixing copper with your fungicide applications. Find information about reducing bacterial diseases here: http://extension.psu.edu/plants/vegetable-fruit/news/2015/farming-like-you-expect-bacterial-diseases
TOMCAST uses leaf wetness and temperature data to calculate disease severity values (DSV’s) as shown in Table 1.
TOMCAST is derived from the original F.A.S.T. (Forecasting Alternaria solani on Tomatoes) model developed by Drs. Madden, Pennypacker, and MacNab ? at Pennsylvania State University (PSU). The PSU F.A.S.T. model was further modified by Dr. Pitblado at the Ridgetown College in Ontario into what we now recognize as the TOMCAST model used by Ohio State University Extension.
DSV are A Disease Severity Value (DSV) is the unit of measure given to a specific increment of disease (early blight) development. In other words, a DSV is a numerical representation of how fast or slow disease (early blight) is accumulating in a tomato field. The DSV is determined by two factors; leaf wetness and temperature during the “leaf wet” hours. As the number of leaf wet hours and temperature increases, DSV accumulate at a faster rate. See the Disease Severity Value Chart below.
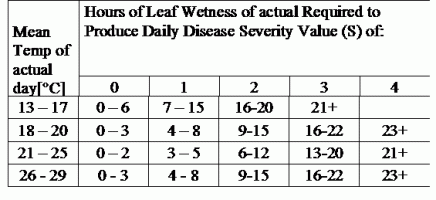
Conversely, when there are fewer leaf wet hours and the temperature is lower, DSV accumulate slowly if at all. When the total number of accumulated DSV exceeds a preset limit, called the spray interval or threshold, a fungicide spray is recommended to protect the foliage and fruit from disease development.

The spray interval (which determines when you should spray) can range between 15-20 DSV. The exact DSV a grower should use is usually supplied by the processor and depends on the fruit quality and end use of the tomatoes. Following a 15 DSV spray interval is a conservative use of the TOMCAST system, meaning you will spray more often than a grower who uses a 19 DSV spray interval with the TOMCAST system. The trade off is in the number of sprays applied during the season and the potential for difference in fruit quality.
USING TOMCAST
Tomatoes grown within 10 miles of a reporting station should benefit from the disease management function of TOMCAST to help forecast early blight, Septoria, and Anthracnose. If you decide to try TOMCAST this season please keep in mind three very important concepts.
One: If this is your first time using the system, it is recommended that only part of your acreage be put into the program to see how it fits with your quality standards and operational style.
Two: Use TOMCAST as a guide to help better time fungicide applications, realizing in some seasons you may actually apply more product than a set schedule program might require.
Three: The further a tomato field is from a reporting site increases the likelihood of distortion in the DSV accumulation, i.e., the reported value may be a few DSV higher or lower than that experienced by the field location. This should be taken into consideration when application of fungicides is likely a few days away. Listen to the DSV reports of nearby stations and triangulate to your own location as the best way to roughly estimate your DSV accumulation.
FIRST SPRAY USING TOMCAST
There has been some discussion over the years regarding the application of the first spray when following TOMCAST. The rule stated in the 1997 Vegetable Production Guide centers around the planting date.
Tomato plants that enter the field before May 20 should have the first spray applied when DSV for that area exceed 25 or when a fail safe date of June 15 arrives. The fail safe is used only if you have not treated since May 20, and is a means to eliminate initial disease inoculum. After the first spray, these tomatoes are subsequently treated when the chosen spray interval (range 15-20 DSV) is exceeded.
Tomatoes planted after May 20 are treated when they exceed the chosen spray interval (range 15-20 DSV) or when they have not been treated by the fail safe date of June 15. Therefore, it is critical to compare the tomato planting date to the date DSV reporting began in that area to guide the spray decision process.
Black leg disease
Disease Cycle
The disease has four main stages on winter oilseed rape:
1. The most important sources of infection for newly-emerged plants are air-borne spores produced on oilseed rape stubble after harvest. Fruiting bodies that produce air-borne spores need about 20 days with rain to mature, eg spores were released early after the wet August in 2005 and in 2006, but late in 2003 when that month was dry.
2. Air-borne spores, released mainly on rainy days, infect leaves to produce the leaf spot stage. Symptoms appear after 5-7 days at 15-20°C but take over 30 days to develop at 3°C.
3. No symptoms are visible while the fungus grows from the leaf spot, down the petiole, to the stem. The growth rate down the petiole may be up to 5mm/day at 15-20°C but slows to 1mm/day at 3-5°C. Fungicides give no control once the stem has been infected.
4. The fungus spreads within the stem, leading to visible stem canker symptoms about six months after leaf infection. Early leaf spotting leads to early stem cankers, which are most likely to reduce yield potential.
Understanding variation
Weather: August and September rainfall is the key factor determining the onset of leaf spotting. Above average rainfall, particularly in August, indicates early risk.
Biology
Leptosphaeria maculans or Phoma lingam survives the intercrop period as mycelium and pseudothecia in crop residues. In Canada, leaf tissue does not persist long enough to allow pseudothecia to develop, but pseudothecia do form on basal stem tissue. Upon maturity, the pseudothecia produce ascospores.
Ascospores of the fungus are released after rainfall when temperatures are between 8-12ºC/46-54ºF. These spores can be wind-dispersed for hundreds of meters (yards). Pycnidia can and do overwinter readily in stubble, but because pycnidiospores are not airborne to any significant extent, they are of minor importance in initiating the first cycle of disease.
Ascospores germinate in the presence of free water from 4-28ºC (40-82ºF). Penetration is through stomates. The pathogen also may be seedborne. Seeds may be infected and/or infested by the pathogen. Infected seeds can give rise to infected seedlings, but levels of seed contamination are always very low. Primary infections usually occur on the cotyledons or basal rosette leaves of the plant. Wet weather favors these primary infections.
The fungus invades the intercellular spaces between the palisade and epidermal layers of the leaf. This symptomless biotrophic phase is followed by invasion of the mesophyll with the resultant death of cells and the appearance of gray-green lesions. The hyphae continue to ramify through the leaf tissue until they reach a leaf vein. The fungus then colonizes the cortex and/or xylem parenchyma of the petiole. At the junction of the petiole and the stem, the fungus invades the stem cortex where it causes a canker. It is at this point that stem resistance is expressed and determines the ability of the disease to proceed to the damaging stem canker phase. Stem cankers form when the plants bolt (produce an upright stem from the rosette on which the flowers form). Stem cankers develop most quickly at 20-24ºC/68-75ºF and are most severe under stress conditions such as mechanical, insect, or herbicide injury.
Pycnidiospores (conidia) are released from the pycnidia under moist conditions in a mucilage, a watery, sticky solution. These spores are responsible for secondary cycles of the disease, but ascospores are the more important source of inoculum because they are more infective and are airborne.
The pynidiospores are dispersed to new infection sites by rain splash. Pycnidiospores germinate more slowly than ascospores and require more than 16 hr of continuous wetness at the optimal temperature range of 20-25ºC/68-77ºF. The minimum latent period (the time from infection to the production of new inoculum) following infection by pycnidiospores is 13 days. Although secondary infections by pycnidiospores do occur, most losses are due to primary infections of leaves by ascospores that lead to basal stem cankers and eventual lodging of the plants.
Model for First Possible Infection in Fall
The development of a phoma stem canker epidemic is seperatet into three stages.
1) In the first stage, the date when phoma leaf spot epidemics start in autumn was predicted from the summer weather data. Since phoma stem canker is a monocyclic disease (one cycle per growing season), the date in autumn when leaf spotting starts to develop is a crucial factor affecting the severity of phoma stem canker epidemics on stems the following summer (West et al. 2001). The date when phoma leaf spotting starts in autumn is estimated from the temperature and rainfall during the intercrop period between the harvest of the previous crop and the establishment of the new crop. Where approximately 4 mm of rain will make the occurence of Black Leg Disease one day earlier and the impact of temperature is higher at the begin of the period in mid summer than in autum.
If the ascospore infection is possible do to the autumn climate, we have to look for the climate needs of ascospore infection.
2) In this second stage we can look for mature ascospores. To mature the ascospores it needs depending on temperature more than 288 hours of air temeprature in between 5 and 25°C and relative humidity higher than 85%. Now it needs 4 mm or rain or more to distribute the ascospores. A leaf wetness period has to start an ascopore infection and if can be completed within 8 hours under optimum temperature.
In later autum and in spring conidia can be formed on mature lesions of Black Leg Disease.
3) In the third stage we have to expect conidia infections, which are started by a leaf wetness period and which are comleted by periods of relative humidity higher than 85% for longer than 8 hours at optimum temperature.
Practical Use of the Black Leg Models
The Black Leg Model starts with the assessment if a Black Leg infection is possible in late summer and early autumn. This part of the model can be used as an negativ prognosis. It has been tested for the UK climate to estimate the first occurence of P. lingam infections. This model is valid for cool and humid climate. It should be used with some caution in continental climate like Hungaria or Austria. The models for Ascospore maturation, ascospore realize and ascospore infection are showing possible ascospore infections during autumn. This models are basing on the biology of the pathogen and will most propably show more ascospore infecitons than you might find in the field. This is because on base of the climate data we do not know anything about the inoculum density and the precrops of the rape fields. Anyhow if one or several ascospore infecitons are fitting to the susceptible plant stages after emergence P. lingam infections will take place and secundary infections from conidia have to be expected during warm and moist periods in fall or spring.
Conidia infections are indicated do to the leaf wetness periods needed for a conidia infection.
Septoria disease
There are two major Septoria diseases in wheat. These are Septoria tritici blotch, incited by the fungus Septoria tritici (teleomorph: Mycophaerella graminicola), and Septoria nodorum blotch, caused by the fungus Septoria nodorum (teleomorph: Leptosphaeria nodorum). Both diseases cause serious yield losses reported to range from 31 to 53 percent (Eyal, 1981; Babadoost and Herbert, 1984; Polley and Thomas, 1991). Worldwide, more than 50 million ha of wheat, mainly growing in the high-rainfall areas, are affected. During the past 25 years, these diseases have been increasing and have become a major limiting factor to wheat production in certain areas. Under severe epidemics, the kernels of susceptible wheat cultivars are shrivelled and are not fit for milling. Epidemics of Septoria tritici blotch and Septoria nodorum blotch of wheat are associated with favourable weather conditions (frequent rains and moderate temperatures), specific cultural practices, availability of inoculum and the presence of susceptible wheat cultivars (Eyal et al., 1987).
Septoria spp. Biology
Following Erick De Wolf, Septoria Tritici Blotch, Kansas State University, April 2008 Septoria tritici blotch known as speckled leaf blotch, is caused by the fungus Septoria tritici. It is distributed in all wheat-growing areas of the world and is a serious problem in many regions. Septoria tritici blotch is most damaging when the disease attacks the upper leaves and heads of susceptible varieties late in the season.
Symptoms
Septoria tritici blotch symptoms first appear in the fall. The initial symptoms are small yellow spots on the leaves. These lesions often become light tan as they age, and the fungal fruiting bodies can be seen embedded in the lesions on the awns. Lesions are irregularly shaped and range from elliptical to long and narrow (Figure 1). Lesions contain small, round, black speckles that are the fruiting bodies of the fungus. The black fruiting bodies look like grains of black pepper and can usually be seen without the aid of a magnifying glass. The disease begins on the lower leaves and gradually progresses to the flag leaf. Leaf sheaths are also susceptible to attack. In wet years, the speckled leaf blotch fungus can move onto the heads and cause brown lesions on the glumes and awns known as glume blotch. These lesions often become light tan as they age and the fungal fruiting bodies are often seen embedded in the lesions on the awns.
The glume blotch phase can cause significant yield loss, but the relationship between disease severity and yield loss is not well understood. Septoria tritici blotch can be confused with other leaf diseases that have very similar symptoms: tan spot and Stagonspora nodorum blotch, for example. It is common for plants to be infected by more than one of these foliar diseases, and it may require laboratory examination to accurately diagnose which diseases are most prevalent. Laboratory examination is nearly always required to distinguish the cause of glume blotch. Knowing the species is not important for spray decisions because all three diseases respond similarly to fungicides. However, knowing which diseases are most prevalent is an important part of variety selection because different genes control the resistance to the diseases.
The most reliable way to distinguish Septoria tritici blotch from the other diseases is by the presence of the black fungal fruiting bodies. The fungus that causes tan spot does not produce this type of reproductive structure. However, under moist conditions, the fungus that causes Stagonospora nodorum blotch will produce light brown fruiting bodies. In addition to the color difference, these structures are also smaller than those produced by Septoria tritici.
Life Cycle
Septoria tritici survives through the summer on residues of a previous wheat crop and initiates infections in the fall. There is some evidence that the fungus is able to survive in association with other grass hosts and wheat seed. These sources of the fungus are probably most important when the wheat residues are absent. Regardless of rotation or residue management practices, there is usually enough inoculum to initiate fall infections. Septoria tritici blotch is favored by cool, wet weather. The optimum temperature range is 16 to 21 °C; however, infections can occur during the winter months at temperatures as low as 5°C. Infection requires at least 6 hours of leaf wetness, and up to 48 hours of wetness are required for maximum infection. Once infection has occurred, the fungus takes 21 to 28 days to develop the characteristic black fruiting bodies and produce a new generation of spores. The spores produced in these fruiting bodies are exuded in sticky masses and require rain to splash them onto the upper leaves and heads.
Infection by Septoria tritici
Pycnidiospores of S. tritici germinate in free water from both ends of the spore or from intercalary cells (Weber, 1922). Spore germination does not begin until about 12 hours after contact with the leaf. Germ tubes grow randomly over the leaf surface. Weber (1922) observed only direct penetration between epidermal cells, but others concluded that penetration through both open and closed stomata is the primary means of host penetration (Benedict, 1971; Cohen and Eyal, 1993; Hilu and Bever, 1957). Kema et al. (1996) observed only stomatal penetration. Hyphae growing through stomata become constricted to about 1 μm diameter, then become wider after reaching the substomatal cavity.
Hyphae grow parallel to the leaf surface under epidermal cells, then through the mesophyll to cells of lower the epidermis, but not into the epidermis. No haustoria are formed and hyphal growth is limited by sclerenchyma cells around the vascular bundles, except when hyphae are very dense. Vascular bundles are not invaded. Hyphae grow intercellularly along cell walls through the mesophyll, branching at a septum or middle of a cell. No macroscopic symptoms appear for about 9 days except for an occasional dead cell, but mesophyll cells die rapidly after 11 days. Pycnidia develop in substomatal chambers. Hyphae seldom grow into host cells (Hilu and Bever, 1957; Kema et al, 1996; Weber, 1922).
Successful infection only occurs after at least 20 hours of high humidity. Only a few brown flecks developed if leaves remained wet for 5-10 hours after spore deposition (Holmes and Colhoun, 1974) or up to 24 hours (Kema et al., 1996). Host-parasite relations are the same on resistant or susceptible wheats. Spore germination on the leaf surface is the same regardless of susceptibility. The number of successful penetrations is about the same, but hyphal growth is faster in susceptible cultivars, resulting in more lesions. Hyphae extend 44 Session 2 — B.M. Cunfer beyond the necrotic area in all cultivars. A toxin may play a role in pathogenesis (Cohen and Eyal, 1993; Hilu and Bever, 1957). In contrast, colonization was greatly reduced on a resistant line (Kema et al., 1996).
Stagonospora (Septoria) and Septoria Pathogens of Cereals: The Infection Process
B.M. Cunfer, Department of Plant Pathology, University of Georgia, Griffin, GA
The infection process has been studied most intensely for Stagonospora (Septoria) nodorum and Septoria tritici. One in-depth study on Septoria passerinii is available. Nearly all of the information reported is for infection by pycnidiospores. However, the infection process for other spore forms is quite similar. The information presented is mostly for infection of leaves under optimum conditions. Some studies were done with intact seedling plants, whereas others were conducted with detached leaves. Infection of the wheat coleoptile and seedling by S. nodorum was described in detail by Baker (1971) and reviewed by Cunfer (1983). Although no precise comparisons have been made, it appears that the infection process has many similarities in each host-parasite system and is typical of many necrotrophic pathogens. Information on factors influencing symptom development and disease expression are excluded but have been reviewed by other authors (Eyal et al., 1987; King et al., 1983; Shipton et al., 1971). A summary of factors affecting spore longevity on the leaf surface is included.
Role of the Cirrus and Spore Survival on the Leaf Surface The most detailed information on the function of the cirrus encasing the pycnidiospores exuded from the pycnidium is for S. nodorum. The cirrus is a gel composed of proteinatous and saccharide compounds. Its composition and function are similar to that of other fungi in the Sphaeropsidales (Fournet, 1969; Fournet et al., 1970; Griffiths and Peverett, 1980). The primary roles of cirrus components are protection of pycnidiospores from dessication and prevention of premature germination.
The cirrus protects the pycnidiospores so that some remain viable at least 28 days (Fournet, 1969). When the cirrus was diluted with water, if the concentration of cirrus solution was >20%, less that 10% of pycnidiospores germinated. At a lower concentration, the components provide nutrients that stimulate spore germination and elongation of germ tubes. Germ tube length increased up to 15% cirrus concentration, then declined moderately at higher concentrations (Harrower, 1976). Brennan et al. (1986) reported greater germination in dilute cirrus fluid. Cirrus components reduced germination at 10-60% relative humidity. Once spores are dispersed, the stimulatory effects of the cirrus fluid are probably negligible (Griffiths and Peverett, 1980).
At 35-45% relative humidity, spores of S. tritici in cirri remained viable at least 60 days (Gough and Lee, 1985). The cirrus components may act as an inhibitor of spore germination, or the high osmotic potential of the cirrus may prevent germination. Pycnidiospores of S. nodorum did not survive for 24 hours at relative humidity above 80% at 20 C. Spores survived two weeks or more at <10% relative humidity (Griffiths and Peverett, 1980). When the cirrus fluid of S. nodorum was diluted with water, about two thirds of the pycnidiospores lost viability within 8 hours, and after 30 hours in daylight, only 5% germinated. When spores were stored in the dark, 40% remained viable after 30 hours (Brennan et al., 1986).
Dry conidia of S. nodorum, shaded and in direct sunlight, survived outdoors at least 56 hours (Fernandes and Hendrix, 1986a). Germination of S. nodorum pycnidiospores was inhibited by continuous UV-B (280-320 nm), whereas germination of S. tritici was not. Germ tube extension under continuous UV-B was inhibited for both fungi, compared with darkness (Rasanayagam et al., 1995).
Infection by Septoria nodorum
The process of host penetration and development of S. nodorum within the leaf was examined in detail by several investigators (Baker and Smith, 1978, Bird and Ride 1981, Karjalainen and Lounatmaa, 1986; Keon and Hargreaves, 1984; Straley, 1979; Weber, 1922). Pycnidiospores tend to lodge in the depressions between two epidermal cells, and many attempted leaf penetrations begin there. Spores germinate on the leaf surface in response to free moisture (Fernandes and Hendrix, 1986b). They begin to germinate 2-3 hours after deposition, and after 8 hours germination can reach 90%. Leaf penetration begins about 10 hours after spore deposition (Bird and Ride, 1981; Brönnimann et al., 1972; Holmes and Colhoun, 1974).
At the onset of germination, the germ tube is surrounded by an amorphous material that attaches to the leaf. Germ tubes growing from either end of a spore and from intercalary cells tend to grow along the depressions between cells and are often oriented along the long axis of the leaf (O’Reilly and Downes, 1986). Hyphae from spores not in depressions grow randomly with occasional branching (Straley, 1979). An appressorium forms with an infection peg that penetrates the cuticle and periclinal walls of epidermal cells directly into the cell lumen, resulting in rapid cell death.
Many penetrations first are subcuticular or lateral growth of a hypha occurs within the cell wall before growth into the cytoplasm (Bird and Ride, 1981; O’Reilly and Downes, 1986). Penetration through both open and closed stomata also occurs and may be faster than direct penetration (Harrower, 1976; Jenkins, 1978; O’Reilly and Downes, 1986; Straley, 1979). Germ tubes branch at stomata and junctions of epidermal cells. Penetration of a germ tube into a stomate may occur without formation of an appressorium. Penetration sometimes occurs through trichomes (Straley, 1979). Apparently, most penetration attempts fail, with dense papillae formed in the cells at the site of attempted penetration (Karjalainen and Lounatmaa, 1986; Bird and Ride, 1981).
After penetration, epidermal cells die quickly and become lignified, and the hyphae grow into the mesophyll. Mesophyll cells become misshapen, and lignified material is deposited outside of some cells, which then collapse. Lignification occurs before hyphae reach the cell. The process is the same in all cultivars but develops more slowly in resistant cultivars. The hyphae grow intercellularly among epidermal cells, then into the mesophyll. When the mesophyll is penetrated, chloroplast deterioration begins in 6-9 days (Karjalainen and Lounatmaa, 1986).
However, the photosynthetic rate begins to decline within a day after infection and before symptoms are visible (Krupinsky et al, 1973). Sclerenchyma tissue around vascular bundles prevents infection of vascular tissue. The vascular bundles block the spread of hyphae through the mesophyll except when sclerenchyma tissue is young and not fully formed (Baker and Smith, 1978).
Stagonospora nodorum releases a wide range of cell wall degrading enzymes including amylase, pectin methyl esterase, polygalacturonases, xylanases, and cellulase in vitro and during infection of wheat leaves (Baker, 1969; Lehtinen, 1993; Magro, 1984). The information related to cell wall degradation by enzymes agrees with histological observations.These enzymes may act in conjunction with toxins. Enzyme sensitivity may be related to resistance and rate of fungal colonization (Magro, 1984). Like many necrotrophs, Septoria and Stagonospora pathogens produce phytotoxic compounds in vitro. Cell deterioration and death in advance of hyphal growth into mesophyll tissue (Bird and Ride, 1981) is consistent with toxin production. However, a definitive role for toxins in the infection process and their relation to host resistance has not been established (Bethenod et al, 1982; Bousquet et al, 1980; Essad and Bousquet, 1981; King et al, 1983). Differences in host range between wheat and barleyadapted strains of S. nodorum may be related to toxin production (Bousquet and Kollmann, 1998). Initiation of spore germination and percentage of spores germinated are not influenced by host susceptibility (Bird and Ride, 1981; Morgan 1974; Straley, 1979; Straley and Scharen, 1979; Baker and Smith, 1978).
Bird and Ride (1981) reported that extension of germ tubes on the leaf surface was slower on resistant than on susceptible cultivars. This mechanism, expressed at least 48 hours after spore deposition, indicates pre-penetration resistance to elongation of germ tubes. There were fewer successful penetrations in resistant cultivars, and penetration proceeded more slowly on resistant cultivars (Baker and Smith, 1978; Bird and Ride, 1981). Lignification was proposed to limit infection in both resistant and susceptible cultivars, but other factors slowed fungal development in resistant lines. In susceptible lines, faster growing Hyphae may escape lignification of host cells.Four days after inoculation of barley with a wheat biotype isolate of S. nodorum, hyphae grew through the cuticle and sometimes in outer cellulose layers of epidermal cell walls. Thick papillae were deposited beneath the penetration hyphae and the cells were not penetrated (Keon and Hargreaves, 1984).
Infection by Septoria passerinii: Green and Dickson (1957) present a detailed description of the infection process of S. passerinii on barley. The infection process is similar to S. tritici. Like S. tritici, the length of time required for leaf penetration is considerably longer than for S. nodorum. Germ tubes branch and grow over the leaf surface at random, but sometimes along depressions between epidermal cells. Leaf penetration is almost exclusively through stomata. Germination hyphae become swollen, and if penetration is unsuccessful, hyphae continue to elongate. No penetration occurs 48 hours after spore deposition. After 72 hours, germ tubes thicken over stomata, grow between guard cells and on urfaces of accessary cells and into the substomatal cavities. Direct penetration between epidermal cells is seen only rarely.
Spore germination and host penetration are the same on resistant and susceptible cultivars. There is much less extension of hyphae within leaves on resistant cultivars and papillae are observed on many but not all cell walls. Hyphae grow beneath the epidermis from one stoma to another, but do not penetrate between epidermal cells. The mesophyll is colonized, but no haustoria form. After the mesophyll cells become necrotic, epidermal cells collapse. Mycelial development in the leaf is sparse and usually blocked by vascular bundles. In younger leaves, if the vascular sheath is less developed, hyphae pass between the bundle and the epidermis. Pycnidia form in substomatal cavities, mostly on the upper leaf surface (Green and Dickson, 1957).
Factors Affecting Spore Longevity on the Leaf Surface Among the Stagonospora and Septoria pathogens of cereals, definitive information on the infection process has been reported only for S. nodorum, S. tritici, and S. passerinii. Like many other necrotrophic pathogens, neither group of pathogens elicit the hypersensitive reaction. A significant difference in the infection process between Septoria and Stagonospora pathogens is that spore germination and penetration proceeds much faster for S. nodorum than for S. tritici and S. passerinii. This has a significant influence on disease epidemiology.
The Septoria pathogens penetrate the plant primarily through stomata, whereas S. nodorum penetrates both directly and through stomata. S. nodorum penetrates and kills the epidermal cells quickly, but S. tritici and S. passerinii do not kill epidermal cells until hyphae have ramified through the leaf mesophyll and rapid necrosis begins. Histological studies of fungal growth following host penetration match the data generated from epidemiological studies of host resistance. Resistance slows the rate of host colonization but has no appreciable effect on the process of lesion development.
The mechanisms controlling host response, whether related to enzymes and toxins or other metabolites released by the pathogens during infection, are still unclear. There is little information about infection by ascospores. The infection process is probably very similar to that for pycnidiospores. Ascospores of Phaeosphaeria nodorum germinate over a wide range of temperatures, and their germ tubes penetrate the leaf directly. However, according to Rapilly et al. (1973), ascospores, unlike pycnidiospores, do not germinate in free water.
Septoria spp. Infection Model
Septoria Infections are possible at low temperatures whereas temperatures below 7°C might not lead to an infections within 2 days. The optimum temperature of the disease is reached in the area of 16 to 21°C. Infections are possible within a period of high relative humditiy or leaf wetness of 14 hours or longer. To meet the conditions we decided to seperate into models for weak, moderate and severe infections. Weak infections can be given if it is possible for the pathogen to infect the host tissue. This means that weak infections can take place if temperatures are in miniumum and leaf wetness periods are of critical duration. A moderate infection will take place under conditions where most infeciton trials lead to reasonable results and severe infections take place under conditions where the pathogen has optimum conditions for infection.

Infection starts after a rain of 0.5 mm. We decided not to use a model for pycnidia formation. The condition needed for pycnidia formation is assumed to be a period with relative humdity higher than 85%. Pycnidia life time is expected to be 24 hours. In all climates where Septoria tritici has a chance to infect we will find 2 hours fulfilling this conditions at nearly every day arround sun rise.
Infection severity evaluation: To be able to assess the Septoria tritici infection pressure in between stage 10 (first leaf trough coleoptile) and stage 32 (node two at least 2 cm above node 1) and in between 32 and 51 (beginning of heading) we have to assess the severity of infections based on climatic conditions. This assesment is done in a 1 to 5 scale. A severity of 1 is given if the condition for a weak infection is fullfiled and it has rained less than 5 mm, otherwise the corresponding sevirity vlaue will be 2. A severity of 3 is given if a moderate infection is fullfilled and it has rained less than 5mm. If it has rained more than 5 mm during a moderate infection or less than 5 mm during a severe infection a severity of 4 is given.
A severe infection with more than 5 mm of rain corresponds with a severity value of 5.
Septoria tritici disease pressure evaluation: The climate is only one factor desciding about the disease pressure in field. The other two factors are the history of the field and the susceptibility of the variety grown. If we can accumulate the disease severity values from stage 10 to stage 32 to value of 4 we can expect a weak disease pressure by the climate. If this value reaches 6 we can expect a moderate disease pressure and if it reaches 10 we can expect a high disease pressure from the climate. Knowing the susceptibility of the variety and the history of the field will lead us to spray or not on a weak or moderate disease pressure in this situation. Having an accumulated value of 10 may lead to a spray in stage 32 anyhow. Decsion of a spray at a later stage is more depending on the spring climate. If we are able to accumulate the severity values since stage 10 to a value of 6 we can expect a weak disease pressure. If this value reaches 10 we can expect an moderate disease pressure and if this value reaches 15 we can expect a high disease pressure form the climate situation.
In FieldClimate we show the Septoria tritici Severity together with the three different infection severities in one graph (see above). Due too rainfall and long leaf wetness periodes conditions for a severe infection by S. tritici have been fullfield on May 14th and 16th. The Severity levels reach the highest value of 5 on May 14th, which means that a high risk for infection is now.
Stagonospora nodorum’s infection biology differs in some extend form this of S. tritici but this difference is not big enough for a seperate model. Therfore we suggest to use this model for the whole complex of Stagnospora and Septoria diseases in cereals including S. passerinii. S. tritici and S. passerinii tend to need longer leaf wetness periods than S. nodorum. In areas with a high pressure of S. nodorum infections classifed to a weak giving a severity value of 2 should be treated more serious then in other areas.
For Septoria nodorum a risk model is shown in FieldClimate (see above). A high risk was determined at the June 17th and July 7th (100%). Depending on the susceptible plant stage for infection plant protection measurements have to bee taken into account if the risk reach 80% (also see wheather forecast, protective plant protection). If the risk was 100% and an infection was already determined systemic plant protection measurements (curative application) has to be taken to protect the plant.
Anthracnose
Several species of plant pathogenic fungi in the genus Colletotrichum cause anthracnose in peppers and many other vegetables and fruits. Until the late 1990s, anthracnose of peppers and tomatoes was only associated with ripe or ripening fruit. Since that time, a more aggressive form of the disease has become established. This form attacks peppers at any stage of fruit development and may threaten the profitability of pepper crops in areas where it becomes established. This disease can also affect tomatoes, strawberries, and possibly other fruit and vegetable crops.
Symptoms
Circular or angular sunken lesions develop on immature fruit of any size. Often multiple lesions form on individual fruit. When disease is severe, lesions may coalesce. Often pink to orange masses of fungal spores form in concentric rings on the surface of the lesions. In older lesions, black structures called acervuli may be observed. With a hand lens, these look like small black dots; under a microscope they look like tufts of tiny black hairs. The pathogen forms spores quickly and profusely and can spread rapidly throughout a pepper crop, resulting in up to 100% yield loss. Lesions may also appear on stems and leaves as irregularly shaped brown spots with dark brown edges.
Pathogen
This form of pepper anthracnose is caused by the fungus Colletotrichum acutatum. The pathogen survives on plant debris from infected crops and on other susceptible plant species. The fungus is not soil-borne for long periods in the absence of infested plant debris. The fungus may also be introduced into a crop on infested seed. During warm and wet periods, spores are splashed by rain or irrigation water from diseased to healthy fruit. Diseased fruit act as a source of inoculum, allowing the disease to spread from plant to plant within the field. The fungus survives in and on seeds. Anthracnose is introduced into the field on infected transplants or it can survive between seasons in plant debris or on weed hosts. Alternative hosts include weeds and other plants in Solanaceae (tomato, potato, eggplant) although infections of these hosts are extremely rare in Florida. Fruit are infected when spores of the fungus or infested debris is rain splashed onto pepper plants. New spores are produced within the infected tissue and then are dispersed to other fruit. Workers may also move spores with equipment or during handling of infected plants. Infection usually occurs during warm, wet weather. Temperatures around 80° F (27° C) are optimum temperatures for disease development, although infection occurs at both higher and lower temperatures. Severe losses occur during rainy weather because the spores are washed or splashed to other fruit resulting in more infections. The disease is more likely to develop on mature fruit that is present for a long period on the plant, although it can occur on both immature and mature fruit. Anthracnose can infect from 15 °C to 30°C. But a long leaf wetness is needed to fullfil the needs for an infection. At optimum temperature from 20°C to 25°C still 12 hours of leaf wetness are needed. Higher or cooler temperature will need even longer leaf weetness periods (no linear function/array needed for calculation). FieldClimate calculates the possible infection events on base of leaf wetness and the temperatures during this event.
Downy mildew
Biology of Plasmopara viticola (Downy Mildew)
P. viticola is an obligate parasite. Which means that green, fresh vine organs are needed to grow. During the vegetation free period it persists forming fruiting bodies, so called oospores. Oospores of oomycetes can survive very long periods in soil. Therefore, we can find downy mildew in places where infections are not possible in every year. In spring when the top soil is moist and warm enough, the oospores will form so called macrosporangia which can release up to 200 zoospores into free water. The zoospores move up to the leaves and clusters by wind in water droplets. They do have two flagella and they move in a water film on the downside of the leaves or the clusters and young berries to find a stoma to enter plant tissue. They enter and germinate into the stoma, in which they transfer all their plasma within less than one hour. In microscopic studies, the finding of stoma, encystation and the germination into the stoma was finished within 90 minutes.
P. viticola grows in the intercellular space and it feeds itself with haustorias penetrating the epidermic and parenchymal cells. In dependence of temperature and relative humidity it develops enough intercellular growth with enough haustorias to form a substomatel body which fills up the whole substomatel area and which lifts up the epidermal tissue from the parenchymal tissue. This leads to the visible symptom of the oil spot.
Oomycetes are sporulating in the absence of light when relative humidity is very high. In P. viticola there is no sporulation if temperatures are below 12°C and relative humidity is below 95%. Sporangiaphores are formed by the substomatel vesicles and they will come out of the stoma. The fresh formed sporangia are sticky and can only be removed from the sporangiaphores by water. During the decrease of relative humidity, the sporangia become try and could be removed by wind too.
Sporangia will release up to 20 zoospores into free water. These zoospores have to be distributed by wind in water droplets too to come to fresh leaves, or the sporangia can be distributed by rain or wind itself. The infection process of primary and secondary infection is the same.
Do to the big importance of the sexual stage for the hibernation of the pathogen we can assume mating types fitting in all vineyards where grape vine downy mildew occurs. The zoospore formation takes place on older leaves during summer and early autumn.
Primary Infection in detail
Infections coming from the oospores are called primary infections. This term is misleading since several primary infections can occur in early summer. In epidemiological events, the primary infection does not play an important role if there are sufficient oil spots in the vineyards and the infection potential of the summer spores (sporangia) exceeds the oospores.
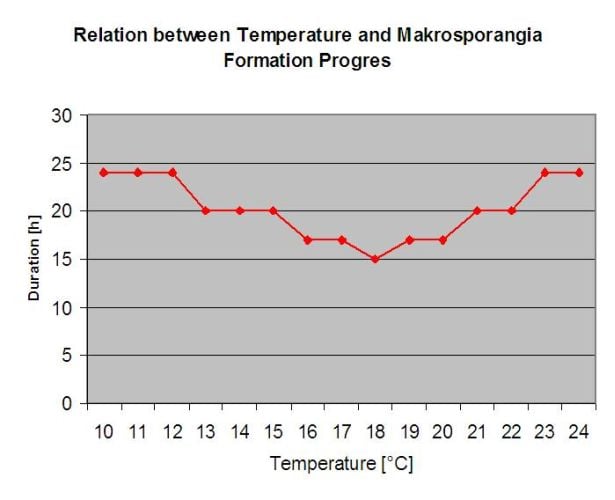
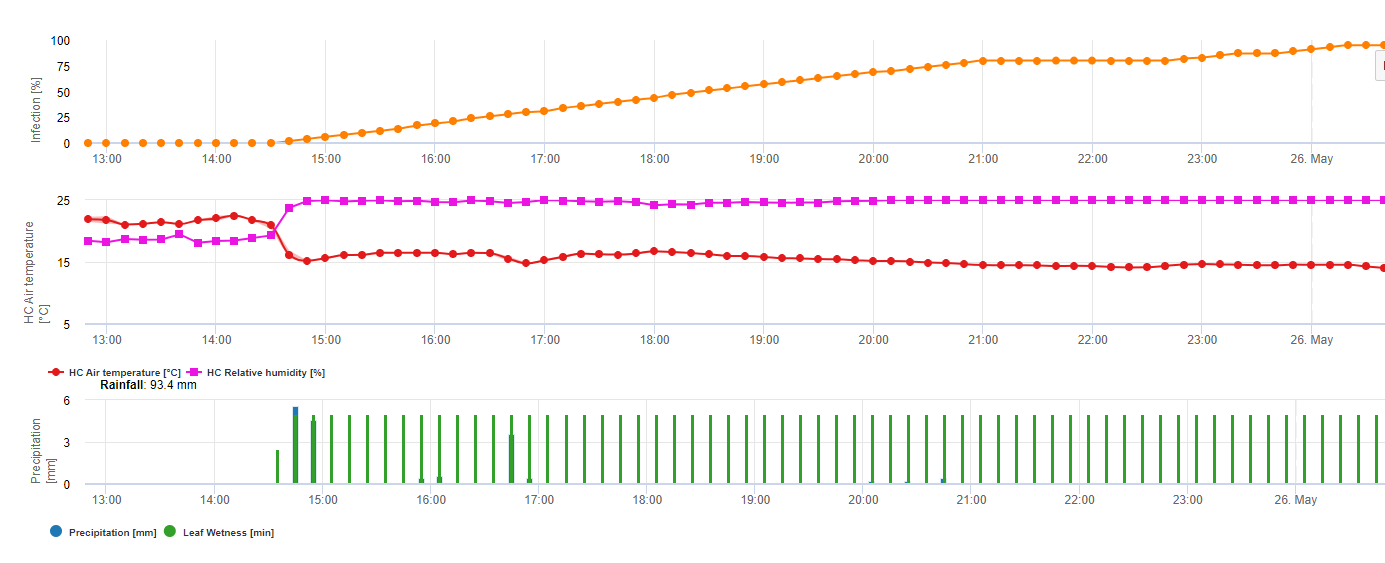
The overwintering oospores develop to so-called macrosporangia’s whenever there is sufficient relative humidity for about 24 hours. The macrosporangia’s release their zoospores into free water and heavy rain (as in a thunderstorm). Spores are carried to the vine leaves and/or green shoots. Primary infections, therefore, need longer rainy periods or several successive thunderstorms. The first rain causes the fallen leaves to be saturated with water and strong rain on the following days causes the macrosporangia’s to release their zoospores, which reach the vine leaves or shoots. For the successful infection, a sufficiently long leaf wetness period is necessary to allow the zoospores to reach the stoma of the leaves or shoots and infect them (the Illustration above shows the development cycle of the downy mildew of the vine (Plasmopara viticola).
The Model for the Downy Mildew primary infection checks first, if the weather is suitable for development of macrosporangia’s. This is the case as long as the leaves are wet, or the relative humidity after the rain does not fall below 70%. Depending on the temperature, the macrosporangia’s can develop within 16 to 24 hours. If mature macrosporangia’s are available, it will be shown graphically in the display of the Downy Mildew primary infection. If macrosporangia’s are present, a strong rainfall can spread their zoospores. A continuous rain of 5 mm is interpreted as a strong rain and the zoospores are spread, a primary infection is than possible if the leaf wetness has lasted long enough.
Secondary Infections
Secondary infections of Plasmopara viticola are only possible if already mature oil spots exist in or nearby your vineyard. Oil spots are mature when they are able to sporulate (produce sporangia). Sporangia are only produced by night. Sunlight inhibits the sporulation. Sporangia are produced if it is warmer than 12°C and the relative humidity is very high. The rate of sporangia production increases with temperature up to 24°C. The optimal temperature for sporulation on European grape varieties (Vitis vinifera) is about 24°C. If temperatures exceed 29°C, no sporulation can take place. In our model we check if humidity of more than 95% occurs during the night. If this condition lasts for an accumulated hourly temperature of more than 50°C, the sporulation finished and new sporangia of Plasmopara exist in the vineyard. For example, 50°C hours correspond to 4 hours with 13°C or 3 hours with 17°C.
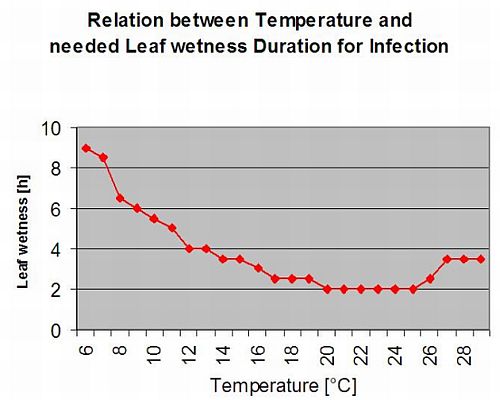
The following graph show the relation between temperature and wet conditions (relative humidity, leaf wetness, precipitation) which are used to model the infection events in fieldclimate.com.
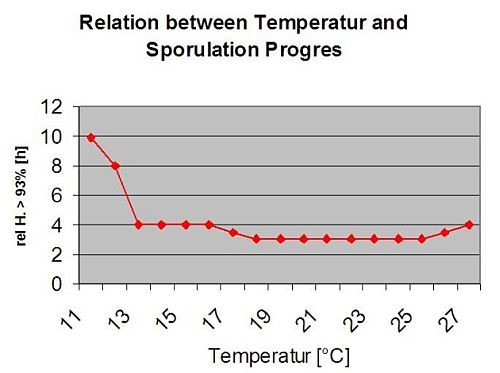
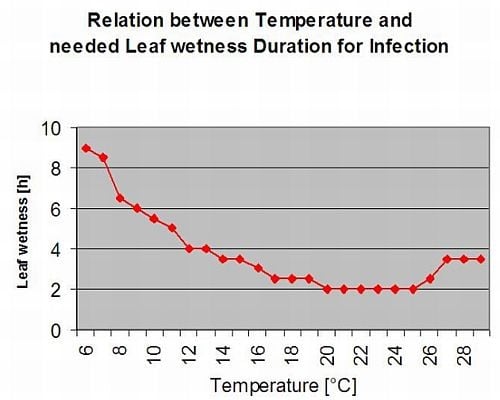
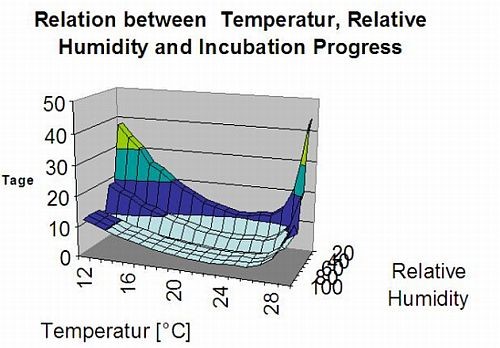
Sporangia of Plasmopara viticola have a limited lifetime. The warmer it is and the drier the air, the faster they die (in ng.fieldclimate.com we reset to 0 when r.h. is below 50%). They definitely die in the next dew or leaf wetness period, which is too short for infection. The fitness of older sporangia is quite limited therefore. Our model assumes the lifetime of sporangia to be limited to one day.
In order to cause new infections, the sporangia must be distributed. There are two ways for distributing spores: If it rains immediately after the forming of sporangia, they spread with the rain splashes. If the vine leaves stay long enough wet, a high level of Plasmopara viticola infections takes place. If the next morning begins without rain and with decreasing humidity, the dried up sporangia abscise from their branches. Even a slight air movement will carry them to healthy leaves. Unless it rains soon, they will die.
Downy Mildew Primary Infection
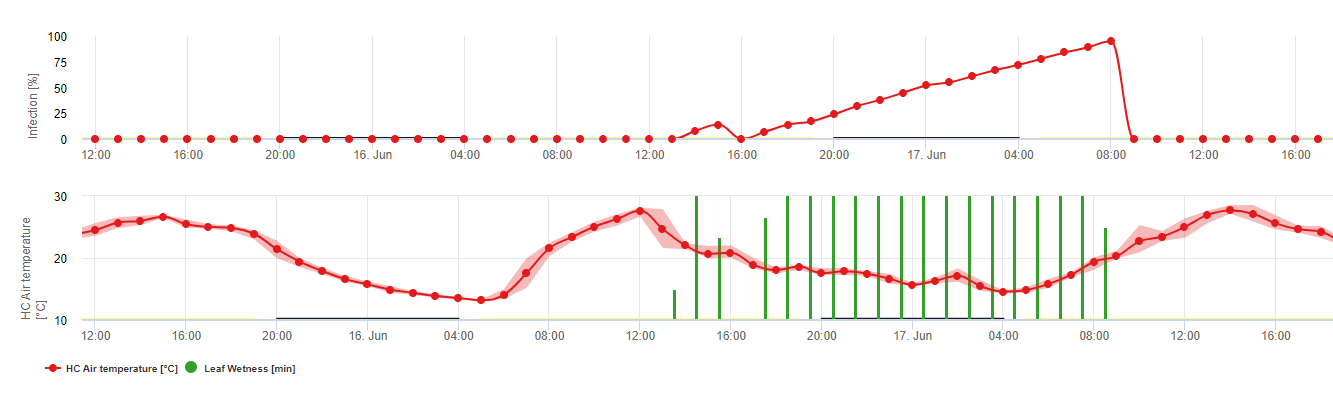
Infections are shown after macrospore development (orange, fifth graph) in three different severity classes (weak: orange, moderate: green, severe: red) depending on the rain amount and therefore distribution of spores in the vineyard. If 100% infection is determinated optimal conditions have been given to enter the plant tissue (plant protection curative) and the incubation curve (first graph) starts to increase. If the incubation finish (100%) and no plant protection has been taken into account symptoms (oil spots) should have been checked in the field.
1. The infection progress curve and after full infection the consequent incubation period (time between infection and visible symptoms in the field).
2. An infection progress curve is determined when macrosporangia or sporangia are present and leaves are wet. It increases with ongoing leaf wetness and temperature. If the infection progress curve reaches 100% the infection can be assumed to be completed. The incubation progress curve which belongs to the specific infection is started to be calculated with the start of an infection. If an infection can not be completed, the incubation curve will be stopped too.
3. The weather conditions are displayed on the same page with the disease model results. Therefore, you can check with one glance for temperature, relative humidity, rain and leaf wetness during the infection.
4. The model is pointing out infection dates for primary and secondary grape vine downy mildew infections. This is the most critical information. Infections, which have not yet been covered by either preventative or curative fungicides, can be disastrous for the yield/ the quality. Further on information over infection severities (weak/moderate/severe) could be helpful to decide plant protection strategy in dependence of the history of vineyard (amount of inoculum), variety and experience the years before.
Plant protection strategies
- Prophylactic and systemic or curative fungicides are widely used for the control of downy mildew. Prophylactic chemicals are applied before, but as close as possible to an infection event in the period of greatest host susceptibility, between shoot length of 10 cm and pea-sized berries.
- Prophylactic chemicals maybe used also by spraying as close as possible before a forecast weather event of more than 2mm rain (for primary circle).
- Curative fungicide should be applied as soon as possible after an infection event (100% infection) and before oilspots appear. Be aware of preventing the development of fungicide resistance.
- Monitoring of the vineyard for the presence of downy mildew (oilspots) should be done regularly and taking at least 200 vines into account. A risk is considered, if more than two oilspots per 50 vines are seen.
The model bases on the publications of MUELLER-THURGAU, ARENS, MUELLER and SLEUMER, BLAESER, HERZOG, GEHMANN and many other workers connected to research network on grape vine downy and powdery mildew epidemiology since the early nineteenth of the last century.
Powdery mildew
Tomato powdery mildew may be caused by three pathogens worldwide.
Leveillula taurica (Oidiopsis taurica) is a pathogen of a wide range of host species in warm arid to semiarid climates in Asia, the Mediterranean, Africa, and more recently the southwest United States.
Erysiphe orontii (E. cichoracearum and E. polyphaga) is another species common to many host plants in both temperate and tropical regions.
And as a third species Odium lycopersicum.
Factors for Disease Development
- relative humidity levels > 50% (optimum RH > 90%)
- free water on leaf surfaces is not necessary
- temperature range: 10-35 °C (best below 30 °C)
Powdery mildew is an inoculum driven disease. Therefore just risky periods could be determined, main factor for the damage, e.g. the outbreak is the initial inoculum (which is active in a broad range of temperature). So for control stragies: combine the modeling of the risky period together with the monitoring of fungal inoculum (disease) in the field!
Open Field Tomato
Asparagus rust
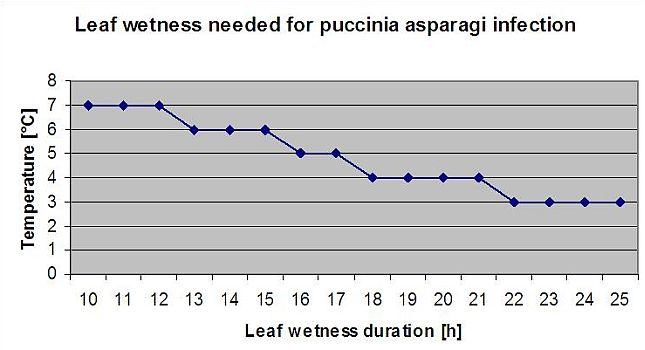
Asparagus rust Puccinia asparagi is favoured by moist and warm climate. Small amounts of rain and wind will distribute the urediaspores whereas heavy rains can wash them down to soil. By this reason a leaf wetness period with more than 10 mm of rain is not taken for an infection periods. Leaf wetness periods coming from light rains and dews from 3 to 8 hours with temperatures from 25°C down to 10°C can be infection periods for urediaspores in summer and late summer.
Stemphylium leaf blight
Stemphylium leaf blight is caused by the fungus Stemphylium vesicarium. Small, light yellow to brown and water-soaked lesions develop on leaves. These small lesions grow into elongated spots that frequently coalesce resulting in blighted leaves. Lesions usually turn light brown to tan at the center and later dark olive brown to black as the spores of this pathogen develop. S. vesicarium normally invades dead and dying onion tissue, such as leaf tips, purple blotch and downy mildew lesions, injured tissue, and senescent tissue. Infection usually remains restricted to leaves and does not extend into the bulb scales. Lesions generally occur on the side of the leaf facing the prevailing wind. Long periods of warm wet conditions encourage disease development.
Stemphylium versicarium model is based on the work of SUHERI and PRICE in onions and LLorent, VILARDELL, BUGIANI, GHERARDI and MONTESINO in pear. Infection curves for weak, moderate and severe infections are computed. It depends on the disease history of the location wheather a treatment has to be taken into consideration by a weak, moderate or heavy infection.
With this separation into favourable, moderate favourable and very favourable situations it is up to the grower to decide how big the disease pressure in a specific field will be and if he has to cover a specific infection.
Temperature: 11-30°C
Leaf wetness: > 0 (start for infection) or rel. humidity > 90% (just if there was already an infection before calculated and is still holding)
light infection (11-30°C), moderate infection (13-30°C), severe infection (16-30°C), array with leaf wetness duration (see above)
Reset: if rel. humidity is lower than 70%.
S. versicarium infections exists often together with Alternaria porri infections. Therefore the TomCast (Alternaria) model is quite frequently used for both.
Cercospora leaf spot
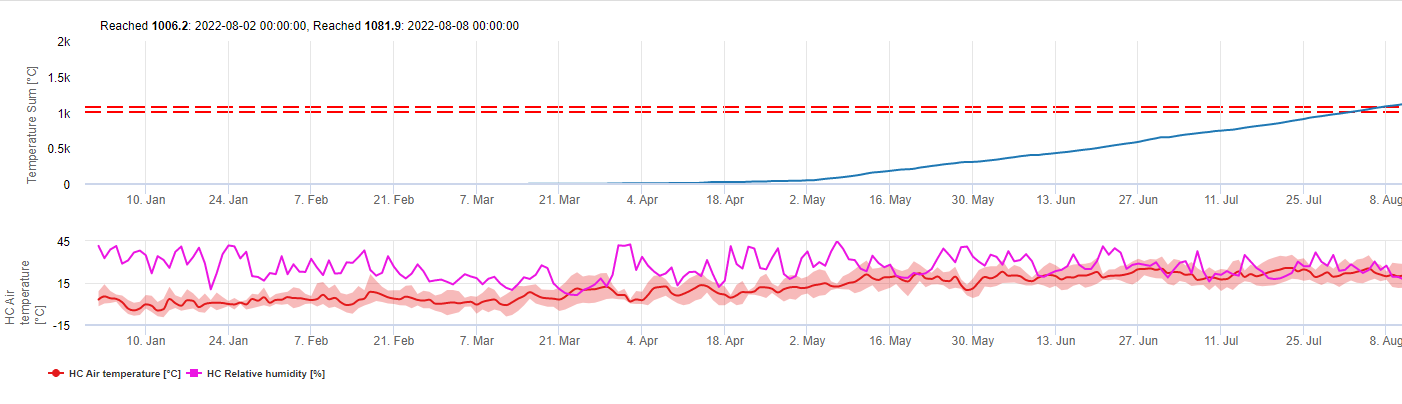
This model estimates the date of the first occurrence of C. beticola on base of the accumulated daily average temperatures since the first of January over 5°C. It takes threshold of 1006.2°C in periods with relative humidity higher than 60% and if we would have no relative humidity a threshold of 1081.9°C is valid.
This model have been developed for Italy and is used in Italy and Germany.
For the year 2010 in Styria CercoPrim indicates the 19th of June for the first spray. This is approximately the same date the Pessl Instruments Risk model or the DIV model would have indicated to spry susceptible varieties.
Purple spot disease
The Pathogen
Purple spot disease on asparagus spears and fern is caused by the fungus Stemphylium vesicarium. The fungus survives the winter as sexual spores (ascospores) in a sac (ascus) produced in overwintering structures (pseudothecia) that appear as small black dots on asparagus debris from the previous season. The ascospores are released from the ascus by rain and can be carried by the wind to newly emerged asparagus plants, where they cause the primary infection of the growing season. These new infections result in spores (conidia) produced by an asexual process, which in turn can cause secondary infections, a process that is repeated as long as temperatures and rainfall are favorable.
The Disease
The emergence of purple spot as a significant problem in the production of asparagus in Michigan may be due to the adoption of a no- till cultural system, whereby the dried fern from the previous season is chopped in April and left on the soil surface. This residue persists through the harvest season (mid-June) and is visible through the fern growth period (late June to September) and is the source of ascospores which start infections early in the growing season. Symptoms of the disease are: The disease appears as numerous, slightly sunken, purplish spots with brown centers occurring on harvested spears and fern. Lesions on spears are often found on the windward side, because blowing sand causes wounding which favours infection. During epidemic years spotting can occur on 60-90% of the spears and may result in rejection of the crop, especially for fresh market sales. Spots also occur on the asparagus ferns, affecting the main stem, secondary branches and needles (cladophylls). Severe infection of the fern can result in premature defoliation of the plant. Increase in the severity of purple spot disease is associated with extended periods of rainfall, fog or dew.
Text credit: Mary K. Hausbeck, Professor and Extension Specialist, Michigan State University, Department of Plant Pathology, E. Lansing
TomCast for Asparagus
Background: TOMCAST (TOMato disease foreCASTing) is a computer model based on field data that attempts to predict fungal disease development, namely Early Blight, Septoria Leaf Spot and Anthracnose on tomatoes. Field placed data loggers are recording hourly leaf wetness and temperature data. This data where analysed over a 24 hour period and may result in the formation of a Disease Severity Value (DSV); essentially an increment of disease development. As DSV accumulate, disease pressure continues to build on the crop. When the number of accumulated DSV exceed the spray interval, a fungicide application is recommended to relieve the disease pressure.
TOMCAST is derived from the original F.A.S.T. (Forecasting Alternaria solani on Tomatoes) model developed by Dr. Madden, Pennypacker, and MacNab at Pennsylvania State University (PSU). The PSU F.A.S.T. model was further modified by Dr. Pitblado at the Ridgetown College in Ontario into what we now recognize as the TOMCAST model used by Ohio State University Extension.
DSV are: A Disease Severity Value (DSV) is the unit of measure given to a specific increment of disease (early blight) development.
In other words, a DSV is a numerical representation of how fast or slow disease (early blight) is accumulating in a tomato field. The DSV is determined by two factors: leaf wetness and temperature during the “leaf wet” hours. As the number of leaf wet hours and temperature increases, DSV accumulate at a faster rate. See the Disease Severity Value Chart below.
Conversely, when there are fewer leaf wet hours and the temperature is lower, DSV accumulate slowly if at all. When the total number of accumulated DSV exceeds a preset limit, called the spray interval or threshold, a fungicide spray is recommended to protect the foliage and fruit from disease development.
The spray interval (which determines when you should spray) can range between 15-20 DSV. The exact DSV a grower should use is usually supplied by the processor and depends on the fruit quality and end use of the tomatoes. Following a 15 DSV spray interval is a conservative use of the TOMCAST system, meaning you will spray more often than a grower who uses a 19 DSV spray interval with the TOMCAST system. The tradeoff is in the number of sprays applied during the season and the potential for difference in fruit quality.
 TomCast with Asparagus
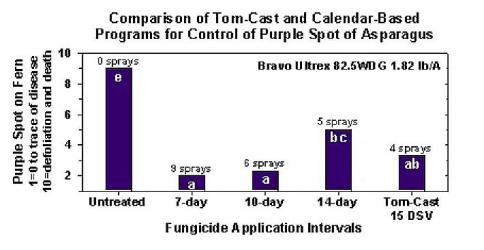
TomCast with Asparagus
Research has determined that the Tom-Cast disease forecaster is a promising alternative to calendar-based spraying of fern in commercial asparagus fields. Tom-Cast alerts growers when the environmental conditions are favourable for purple spot disease development (extended dew or rainy periods accompanied by warm temperatures). Effective fungicides applied according to the Tom-Cast disease forecaster allows growers to manage purple spot disease of asparagus, while saving money and preserving the environment.
Text credit: Jim Jasinski, TOMCAST Coordinator FOR OHIO, INDIANA, & MICHIGAN
Purple spot Infection Model
The Stemphylium versicarium infection model is based on the work of SUHERI and PRICE in onions and LLorent, VILARDELL, BUGIANI, GHERARDI and MONTESINO in pear.
Infection curves for light, moderate and severe infections are computed.
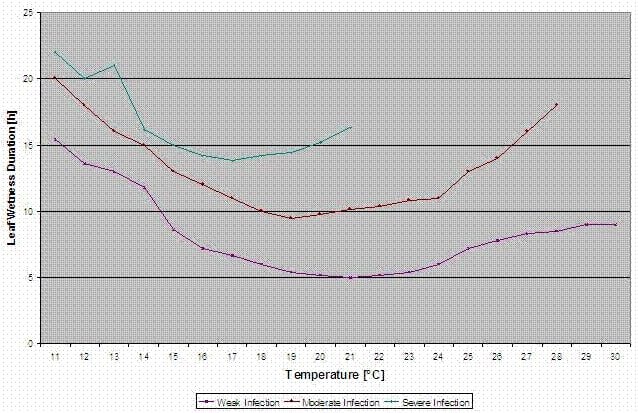
With this separation into favourable, moderate favourable and very favourable situations it is to the grower to decide how big the disease pressure in a specific field will be and if he has to cover a specific infection.
Conditions:
Temperature: 11-30°C
Leaf wetness > 0 (start for infection) or rel. humidity > 90% (just if there was already an infection before calculated and is still holding)
light infection (11-30°C), moderate infection (13-30°C), severe infection (16-30°C), an array with leaf wetness duration (see above)
Reset: if rel. humidity is lower than 70%.
Recommended equipment
Check which sensor set is needed for monitoring this crop’s potential diseases.