
Grapevine disease models
Downy Mildew
Pathogen
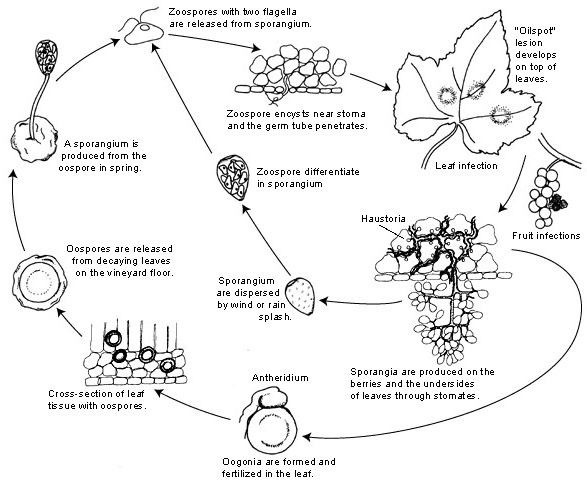
The pathogen for Downy Mildew in grapes is Plasmopara viticola. It is an obligate parasite, meaning that green and fresh vine organs are needed for growth. It has both asexual and sexual reproductive stages —zoospores and oospores. Hence, there are two stages of infection: primary and secondary infection.
Primary infection begins as overwintering oospores germinate, forming sporangia in spring when the weather becomes warm. Sporangia are produced by night as sunlight inhibits sporulation. The thick-walled oospores help the pathogen survive harsh winter conditions and initiate primary infection. Sporangia are dispersed by either rain or wind and release zoospores in free water. For successful infection, sufficiently long leaf wetness or rainfall is necessary.
Secondary infection is only possible in the presence of mature oil spots and occurs by zoospores and sporangia. Zoospores and sporangia are highly sensitive to low humidity and light, which shortens their viability, meaning that most infection happens soon after release. Zoospores penetrate plant tissues via germ tubes and develop hyphae for further infection. These hyphae form oily lesions after the incubation period that ranges from 5 to 21 days depending on temperature – the lower the temperature, the longer it takes.
As the weather gets cold again, which is unfavorable for the pathogen, it shifts to sexual production, producing oospores. These oospores remain dormant during winter, and the cycle repeats.
Symptoms
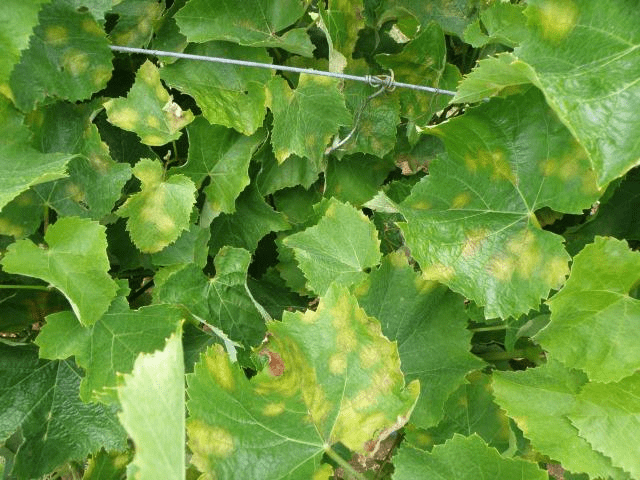
Small yellow lesions appear on the upper surface of leaves, sometimes surrounded by brownish halos. Oil spots expand and integrate as maturation and halos fade away. Sporangia later produce a white-cottony mildew on the underside of lesions and necrosis follows. Oily brown areas also appear on shoots, stems, and berries. Under warm humid nights, they may get covered with white down due to sporulation. Severe infection causes premature fall.
FieldClimate Models
- There are two models in FieldClimate – primary and secondary infection.
- The first graph displays the incubation time after an infection has been completed. The lower graphs depict the infection progress for a weak, moderate, and severe severity level. An infection has to be assumed when the increasing curve in the graph reaches 100% – spraying can be considered if a serious level of infection is shown.
Primary Infection
Sensors needed:
- Air temperature
- Relative humidity
- Leaf wetness
- Precipitation
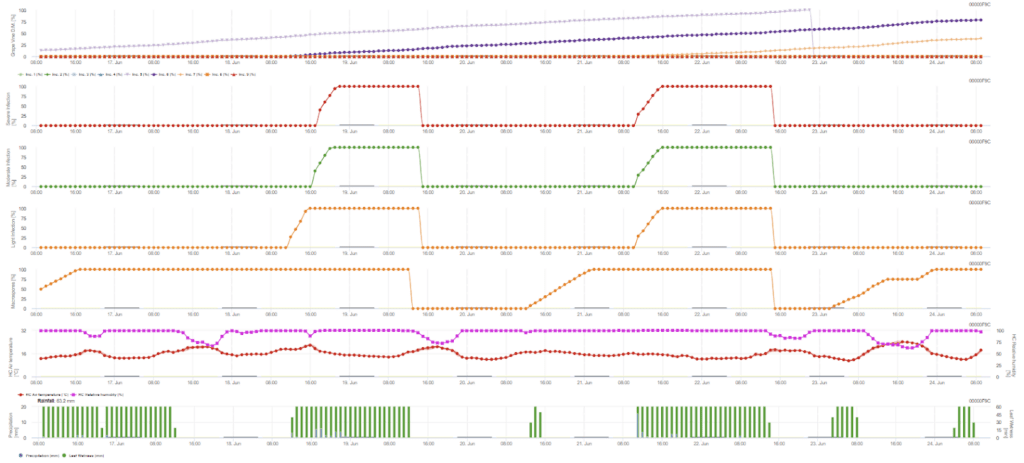
Primary infection checks if the weather is suitable for developing sporangia. This is the case as long as the leaves are wet, or the relative humidity after the rain does not fall below 70%. Sporangia can develop within 16 to 24 hours depending on the temperature. A continuous rain of 5 mm is interpreted as a strong rainfall that can spread zoospores.
Secondary Infection
Sensors needed:
- Air temperature
- Relative humidity
- Leaf wetness
- Precipitation
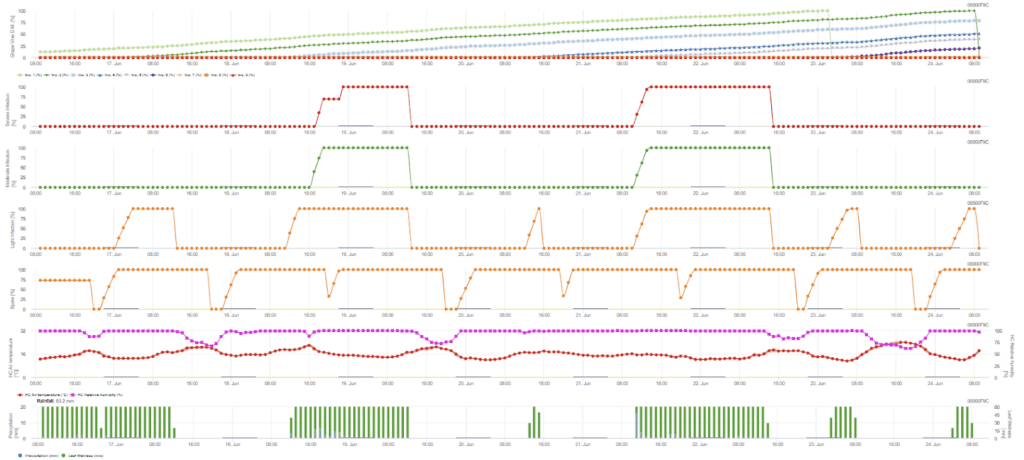
Secondary infection checks if the temperature is warmer than 12 °C and relative humidity is over 95%. The sporangia production rate increases with temperatures up to 23 °C. If this condition lasts for an accumulated hourly temperature of more than 50 °C, we assume that sporulation is finished and new sporangia exist in the vineyard. The accumulated 50 °C corresponds to 4 hours with 13 °C or 3 hours with 17 °C, for example. Sporangia die fast when it gets warmer and drier – when relative humidity falls below 50%, we reset it to 0 and when the temperature exceeds 29 °C, no sporulation can take place.
Literature
- Ash, G. (2000). Downy mildew of grape. The Plant Health Instructor.
- Gessler, C., Pertot, I., & Perazzolli, M. (2011). Plasmopara viticola: a review of knowledge on downy mildew of grapevine and effective disease management. Phytopathologia Mediterranea, 50(1), 3-44.
- Kennelly, M. M., Gadoury, D. M., Wilcox, W. F., Magarey, P. A., & Seem, R. C. (2007). Primary infection, lesion productivity, and survival of sporangia in the grapevine downy mildew pathogen Plasmopara viticola. Phytopathology, 97(4), 512-522.
- Koledenkova, K., Esmaeel, Q., Jacquard, C., Nowak, J., Clément, C., & Ait Barka, E. (2022). Plasmopara viticola the causal agent of downy mildew of grapevine: from its taxonomy to disease management. Frontiers in Microbiology, 13, 889472.
Powdery Mildew
Pathogen
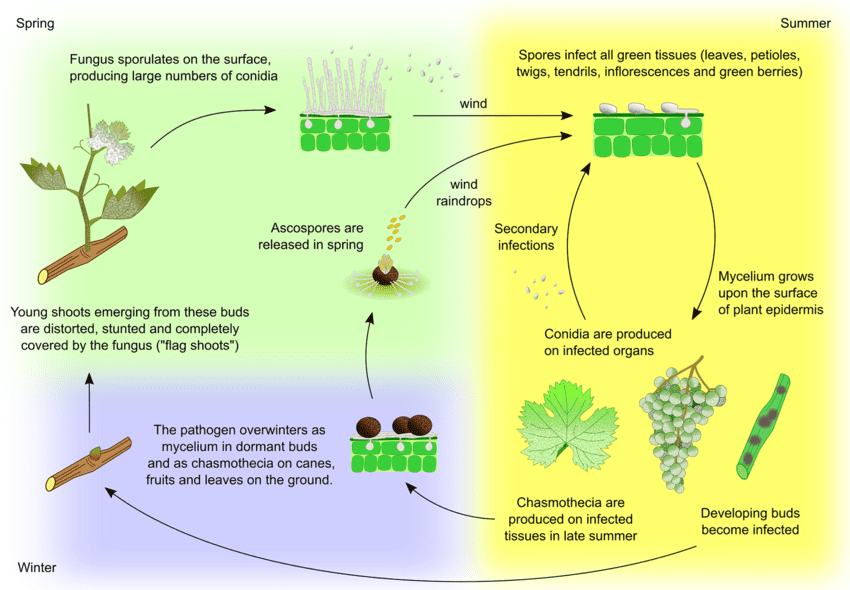
The pathogen for powdery mildew in grapes is Uncinula necator. There are two principal sources of inoculum – overwintering mycelium and ascospores from cleistothecia – which may vary in importance depending on the region.
Mycelium causes flag shoots in spring. Flag shoots are partly or completely mildew-covered shoots formed from latent infected buds. As colonies are formed within, these shoots are well visible and often have deformed leaves.
Cleistothecia release ascospores in early spring. Rainfall plays an important factor for the ascospore release. As temperature rises, dehiscence happens more frequently. Ascospores germinate within 12 hours, forming germ tubes and appressorium on plant tissues, leading to scattered colony formation.
Secondary infection occurs via conidia produced in colonies. Just like ascospores, they germinate and develop germ tubes and appressorium. U. necator needs no free water for infection and no high relative humidity for conidia formation. When weather conditions become less favorable, the fungus forms cleistothecia. It overwinters by either surviving in cleistothecia or as vegetative mycelium in dormant infected buds and the cycle repeats.
Symptoms

Symptoms can be found on all green parts of the vine. Shoots develop colonies and get covered with white-greyish mycelium, known as ‘flag-shoots’. They produce distorted leaves. Mildew colonies are also found on leaves – either on the lower or both sides. Berries develop ash-grey blemishes and get split in severe cases. Canes exhibit dark red-brown patches and may die back from the tips.
FieldClimate Models
Ascospore Infection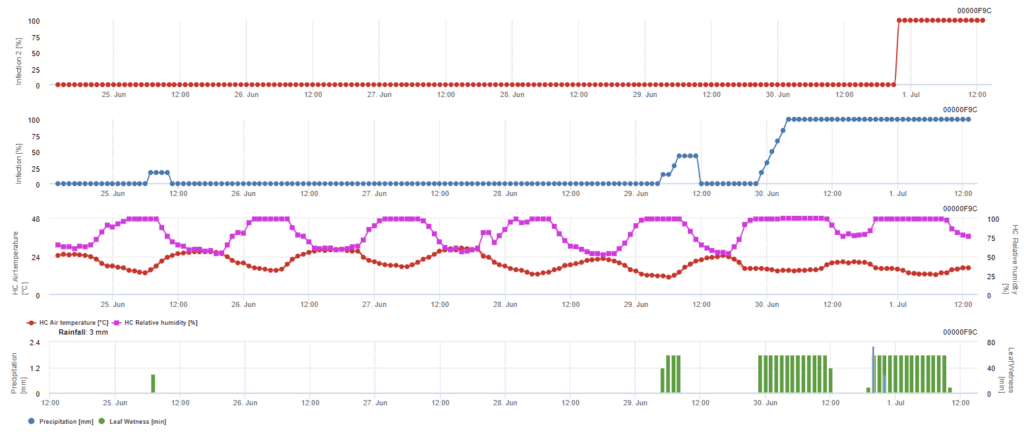
Sensors needed:
- Air Temperature
- Relative Humidity
- Leaf Wetness
- Precipitation
This model is recommended when ascospores are formed during cold winter time (so not infection by mycelium). Ascospore infection predicts the ascospore release and initial infection based on average temperatures during extended leaf wetness periods. For calculation, approximately 2.5 mm of rainfall is required to release ascospores followed by a minimum of 8 to 12 hours of leaf wetness and temperatures between 10 to 15°C. Once the infection has occurred, the model switches to a disease risk assessment phase (Californian Risk model) and is based on the effects of temperature on the reproductive rate of the pathogen.
Californian Risk Model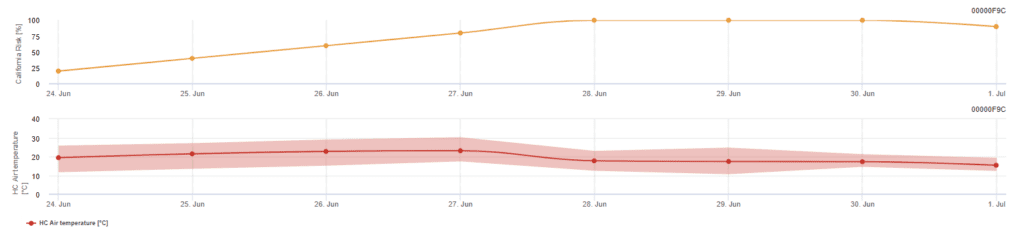
Sensors needed:
- Air Temperature
The model is based on laboratory studies in California. Following ascospore release and germination (model), the subsequent development and reproduction of powdery mildew are influenced by temperatures. It evaluates temperatures and assesses the risk of powdery mildew development using a 0 to 100-point index.
Three days in a row with a minimum of six consecutive hours of temperature between 21 and 30’c is required to initiate the risk assessment index. It gains 20 points for each day that meets 6 or more consecutive hours between 21 and 32°C and loses 10 points for those that don’t do so or when the temperature exceeds 32°C or goes below 21°C.
Low index values of 0~30 indicate that the pathogen is not reproducing. An index of 40~50 is considered moderate and implies a powdery mildew reproductive rate of approximately 15 days. Index values over 60 indicate that the pathogen is reproducing rapidly (every 5 days) and that the risk for a disease epidemic is great.
Pessl Instruments Risk Model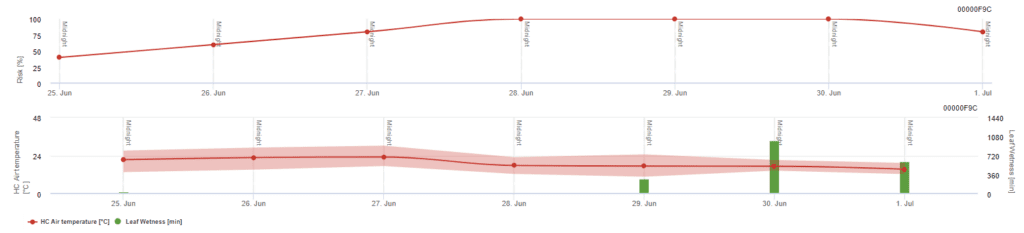
Sensors needed:
- Air Temperature
- Leaf wetness
Besides air temperature, leaf wetness can be a guiding factor as long leaf wetness periods lead to an establishment of antagonistic fungus (Ampelomyces quisqualis), that leads to a decrease of U. necator. The fundamentals of the model are equivalent to the Californian Risk model but leaf wetness is also considered in this model. Leaf wetness longer than 8 hours leads to a decrease of 10 points.
Low index values of 0~20 indicate that the pathogen is not reproducing. An index of 20~60 is considered moderate and a normal spraying interval is valid. Index values over 60 indicate that the pathogen is reproducing rapidly (every 5 days) and that the risk for a disease epidemic is great so shortening the spraying interval is recommended.
Literature
- Gadoury, D. M., & Pearson, R. C. (1990). Ascocarp dehiscence and ascospore discharge in Uncinula necator. Phytopathology, 80(4), 393-401.
- Gadoury, D. M., & Pearson, R. C. (1990). Germination of ascospores and infection of Vitis by Uncinula necator. Phytopathology, 80(11), 1198-1203.
- Hall, T. W. (2000). Epidemiology of grape powdery mildew, Uncinula necator, in the Willamette Valley.
- Halleen, F., & Holz, G. (2001). An Overview of the Biology, Epidemiology and Control of Uncinula Necator (Powdery Mildew) on Grapevine, with Ref Ere Nee to South Africa. South african journal of Enology and Viticulture, 22(2), 111-121.
- Rügner, A., Rumbolz, J., Huber, B., Bleyer, G., Gisi, U., Kassemeyer, H. H., & Guggenheim, R. (2002). Formation of overwintering structures of Uncinula necator and colonization of grapevine under field conditions. Plant pathology, 51(3), 322-330.
Black rot
Pathogen
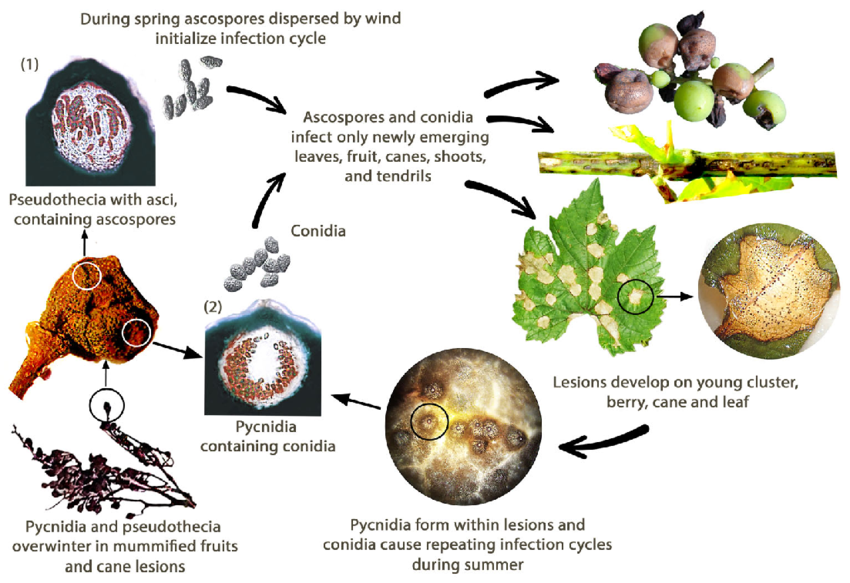
The black rot of grapevine is caused by the fungus Guignardia bidwellii. The fungus overwinters in various parts of the grapevine and can survive for over two years within the lesions of infected shoots.
In spring, pycnidia produce conidia (asexual spores) and pseudothecia generate ascospores (sexual spores). Conidia are typically dispersed over short distances, whereas ascospores can infect over longer distances. These spores are carried by wind and rain to infect young tissues of susceptible hosts, initiating primary infections. Conidia also serve as secondary inoculum, contributing to the rapid and repeated spread of the disease.
During August, the pycnidia transform into an overwintering stage that produces pseudothecia, which generate ascospores. These ascospores are important sources for primary infections in the following spring.
Symptoms

Reddish-brown spots develop that eventually merge, often surrounded by small black dots, which are fungal structures known as pycnidia that produce conidia. Young and rapidly growing leaves are particularly more susceptible to this infection.
Fruit infections typically occur after the calyx has fallen, with most symptoms manifesting when the fruit is half to nearly full size. Initially, small spots emerge, encircled by a brown ring, which then enlarge and darken, ultimately covering the entire berry as the disease develops. Within a few days, infected berries may become mummified, shatter or drop prematurely.
Additionally, other plant parts, such as shoots, stems, and tendrils, are also affected. Oval-shaped lesions that are purple to black develop, and pycnidia become scattered over the surface of these lesions.
FieldClimate Model
Viticulture Black Rot Model
Sensors needed:
- Air temperature
- Relative humidity
- Leaf wetness
- Precipitation
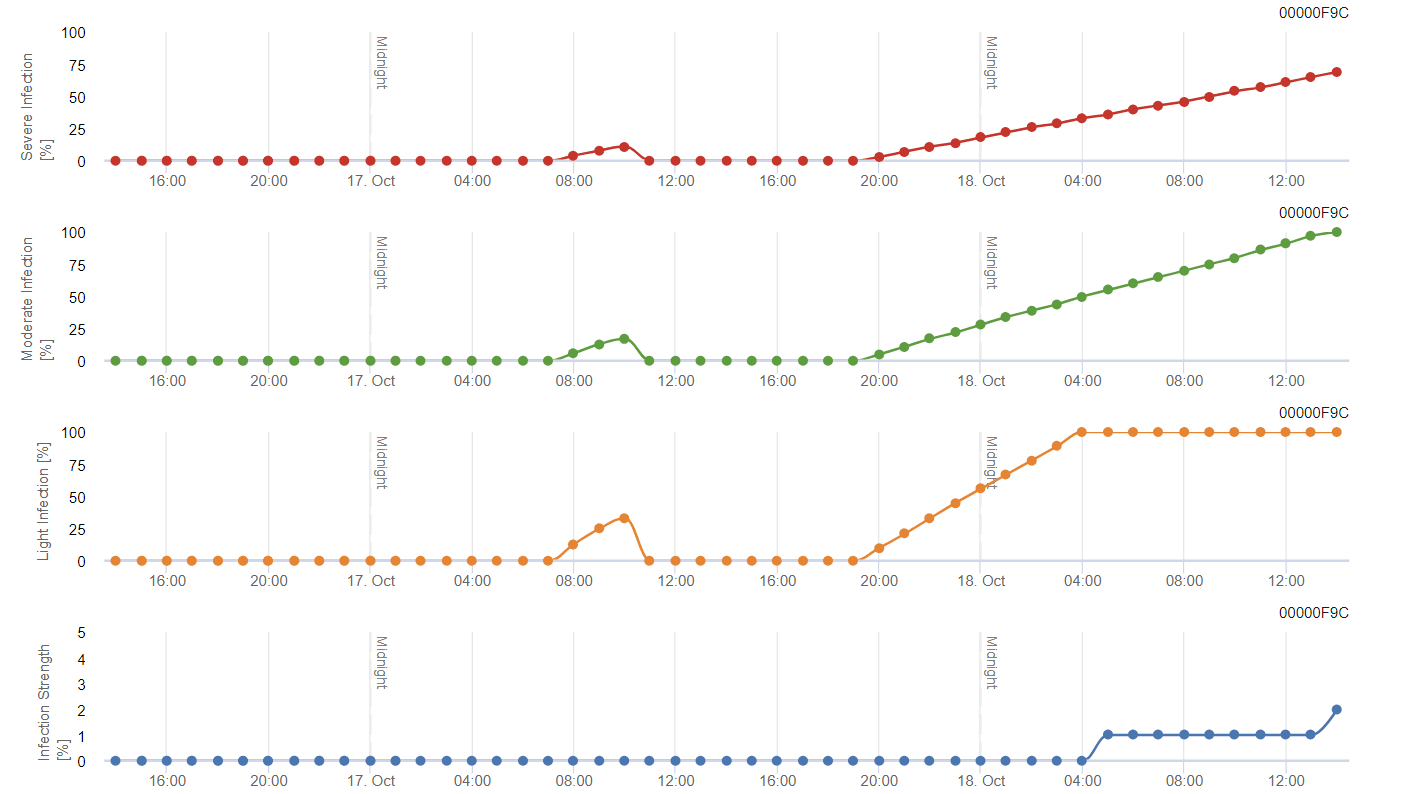
The model is originally based on the literature published by Spotts but with the modification of Daniel Molitor. The revision has introduced three severity classes (light, moderate, and severe) similar to the design of apple scab models. At warm temperatures, the leaf wetness period of 8 hours is enough to favor infections and an infection is assumed to be completed when a graph reaches 100%.
The severity of the infection depends on the time duration of optimal temperature and wetness period of the fungus. Infections meeting the Spotts criteria are rated to be light infections. Infections fulfilling the Spott criteria by 150% and 200% are rated to be moderate and severe infections, respectively.
Depending on the infection pressure, the infections should be covered preventative or a curative spray has to be applied shortly after the infection. In the moderate semi-arid climate of the Mosel or most Austrian wine-growing areas, spraying after determining a light infection would not be recommended.
Literature
- Wilcox, Wayne F. “Black rot Guignardia bidwellii.” Disease Identification Sheet No. 102GFSG-D4. 2003. Cornell. 24 Oct. 2010
- http://www.nysipm.cornell.edu/factsheets/grapes/diseases/grape_br.pdf
- Ellis, Michael A. “Fact sheet Agricultural and Natural Resources: Grape Black Rot.” Department of Plant Pathology. The University of Ohio State Extension. 2008
- http://ohioline.osu.edu/hyg-fact/3000/pdf/HYG_3004_08.pdf
- Molitor, D. (2009). Untersuchungen zur Biologie und Bekämpfung der Schwarzfäule (Guignardia bidwellii) an Weinreben. Gesellschaft zur Förderung der Forschungsanstalt Geisenheim.
- Ries, S. M. (1999). Reports on Plant Diseases: Black Rot of Grape. Integrated Pest Management at the Uni. of Illinois. http://ipm. illinois. edu/diseases/series700/rpd703.
- Spotts, R. A. (1977). Effect of leaf wetness duration and temperature on the infectivity of Guignardia bidwellii on grape leaves. Phytopathology, 67(11), 1378-1381.
- Szabó, M., Csikász-Krizsics, A., Dula, T., Farkas, E., Roznik, D., Kozma, P., & Deák, T. (2023). Black rot of grapes (Guignardia bidwellii)—A comprehensive overview. Horticulturae, 9(2), 130.
- http://extension.cropsciences.illinois.edu/fruitveg/pdfs/771-BlackRotOfGrape.pdf
- https://www.missouribotanicalgarden.org/gardens-gardening/your-garden/help-for-the-home-gardener/advice-tips-resources/insects-pests-and-problems/diseases/fruit-spots/black-rot-of-grapes
Berry moths
Pathogen

Lobesia botrana typically completes two to three generations per year depending on region. The first generation (May and June) only affects the flowers, so treatment is only necessary if moth populations are particularly high. The second generation (July and August) and third generation cause the most damage, with the third generation being especially harmful as it coincides with the ripening of grapes. A fourth generation may occur in warmer climates, but treatment is generally not required as it aligns with the grape harvest.
The moth overwinters as pupae in cocoons, and when temperatures rise in the spring, adults of the first generation emerge, with males typically appearing before females. The flight of the first generation begins near bud break and lasts for 4 to 5 weeks, during which mating occurs. After one to two days after mating, females lay between 80 to 160 eggs.
The eggs, sizing 0.6 to 0.8 mm in diameter, are visible to the naked eye. Initially creamy white, they turn yellow as the embryo develops, with the black color of the larvae’s head. Eggs hatch after 66 degree days Celsius (DDC), approximately 7 to 11 days. Larvae web flower parts together, with development lasting 20 to 30 days. Pupation follows and adults emerge 6 to 14 days later.
The ‘degree-day’ is calculated by measuring how much the daily average temperature exceeds a certain base threshold temperature required for development. As temperatures rise above the base, degree days accumulate.
Adult moths are about 6 to 8 mm long with a wingspan of 11 to 13 mm. Females are slightly larger than males, though both have similar mosaic-patterned wings.
Eggs from the second and third generations hatch faster, within 3 to 5 days. Females lay eggs individually on shaded berries, and upon hatching, the larvae enter berries and hollow them out. In the fall, nights longer than 11 hours trigger diapause, a resting stage that enables the pupae to tolerate cold better, allowing them to overwinter.
Symptoms
The caterpillars of the first generation feed on the vine’s inflorescences, hollowing out flower buds and spinning them for protection, leading to damage within the cluster. In the second generation, larvae target developing berries, creating entry points for fungal pathogens such as Botrytis cinerea. This direct feeding causes visible damage, typically marked by dark spots around the feeding sites. The third generation causes the most significant harm, as the larvae penetrate and feed on ripening berries.
Shriveled berries and larval excrement are left. Shreds of berry epidermal tissue remain loosely attached to the pedicels, along with dry, hollowed-out berry “skin”.
FieldClimate Model
Grapevine Berry Moth Model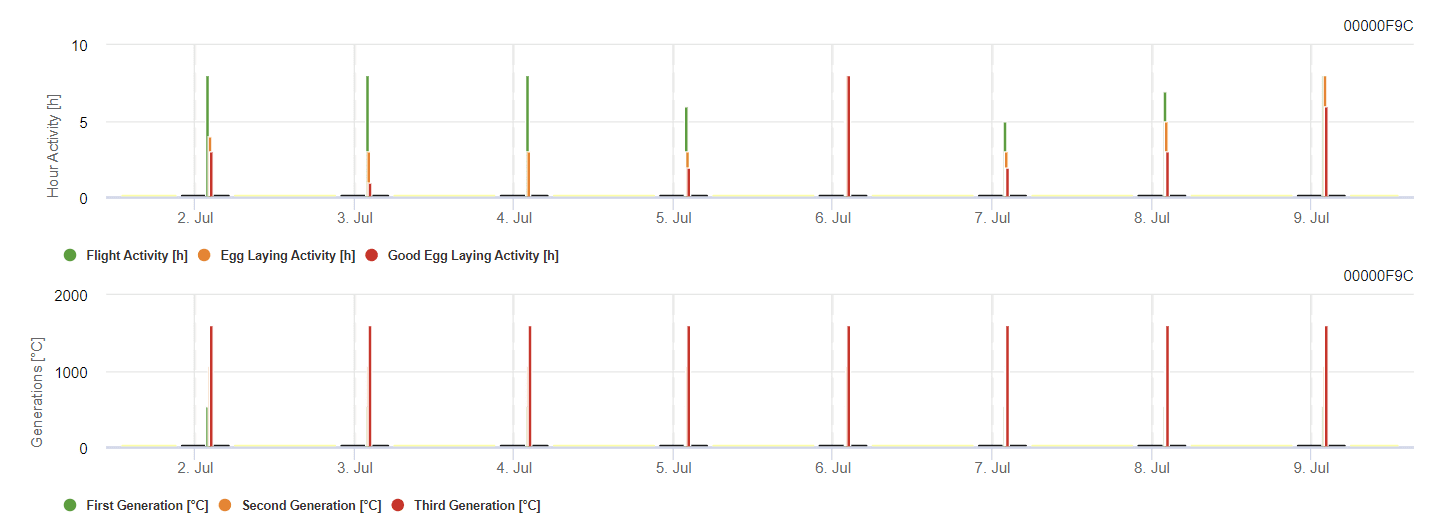
Sensor needed:
- Air temperature
The model calculates the risk by air temperature. The first graph shows flight activity periods with egg-laying and good egg-laying activities for the insect. The second graph shows which generation has to be expected in the period. In the third graph, climatic data are displayed, which show the accumulation of degree days (temperatures > 8°C up to 24°C per hour divided by 24).
Literature
- Varela, L. G., Smith, R. J., Cooper, M. L., & Hoenisch, R. W. (2010). European grapevine moth, Lobesia botrana. Napa Valley vineyards. Pract. Winery Vineyard, 2010, 1-5.
- https://ipm.ucanr.edu/invasive-and-exotic-pests/european-grapevine-moth/
- https://www.ages.at/en/plant/plant-health/pests-from-a-to-z/grape-berry-moth
Grey mould
Pathogen
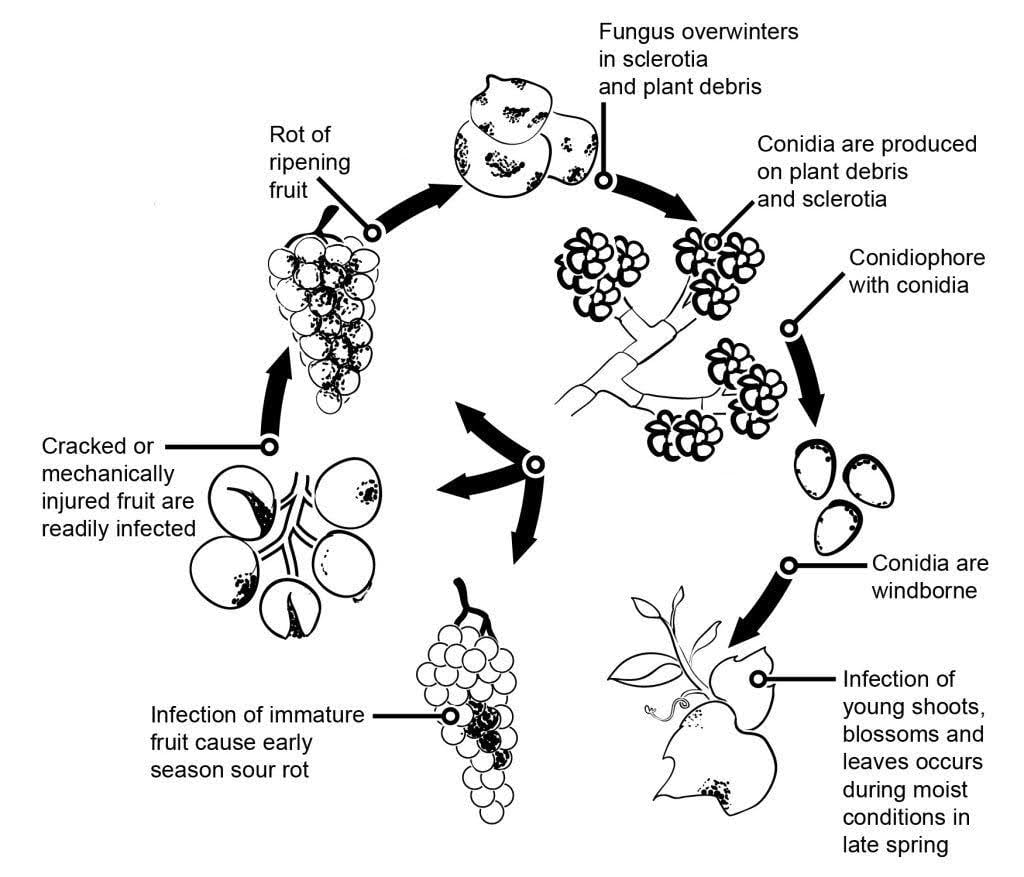
The pathogen of grey mould in grapevine is Botrytis cinerea.
The epidemiology of grey mould is significantly influenced by latent infections initiated by flower infections. Various flower-to-fruit pathways have been identified: The fungus infects the flower’s stylus and reaches the ovule, where it remains latent due to the plant’s preformed defense mechanism. Infections through stamens, petals, and sepals are also critical. B. cinerea can infect stamens and grow systematically toward the receptacle, spreading to the pedicel and vascular tissues in the berries. Other pathways also involve the saprophytic growth of the pathogen.
Overwintering mycelia or sclerotia are the primary sources of infection in spring. Conidia (asexual spores) are produced and dispersed by wind and rain. Upon landing, the conidia germinate, forming germ tubes and appressoria that penetrate and infect the plant. The fungus may remain latent until the fruit ripens and sugar content increases. Conidia generated from primary inoculum sources follow a diurnal cycle of initiation, production, and dissemination.
While the sexual stage of B. cinerea is rarely observed, the pathogen becomes more active as berries ripen. Increased sugar content in the berries increases the susceptibility to infection. In the same way, flowers are highly susceptible when they senesce, with the abundant pollen increasing infection severity.
Symptoms

Grey mould primarily infects ripe grape berries, which initially appear soft and water-soaked. Over time, the berries turn reddish-brown and shrivel. Under favorable conditions, they become covered with grey masses of fungal mycelia and conidia. Healthy berries can also become infected through direct contact with diseased ones.
The fungus may also cause a blossom blight, which can lead to significant crop losses early in the season. Although leaf infections are rare, when they do occur, they start as dull green spots along the veins, which eventually develop into necrotic lesions.
FieldClimate Model
General Grey Mould Model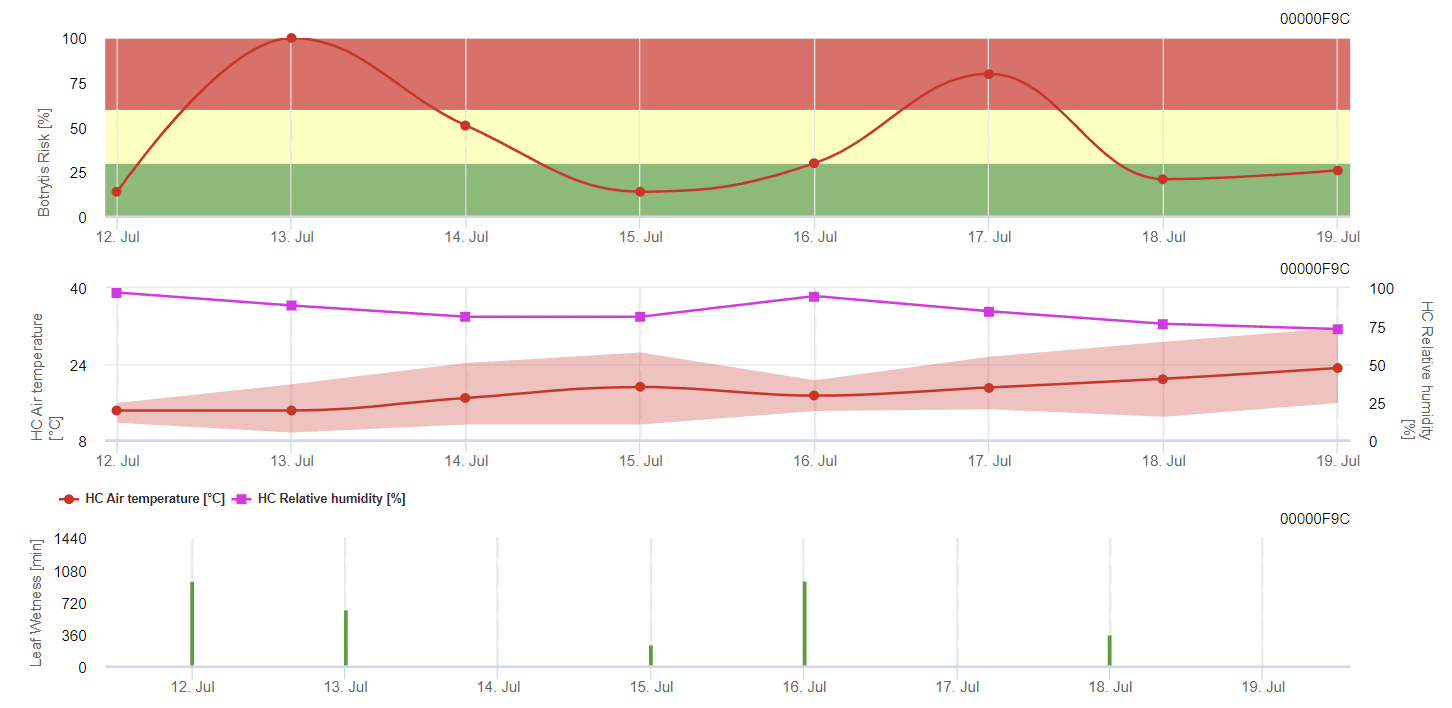
Sensors needed:
- Air temperature
- Relative humidity
- Leaf wetness
B. cinerea is related to moist climate. The fungus requires high relative humidity or the presence of free water for infection. Warm periods with extended leaf wetness period will lead to increased B. cinerea risk while dry periods reduce it. Infection takes place on young shoots during long wet periods or damaging hail storms.
The model calculates the risk in a value of 0 to 100%. This value indicates the pressure of B. cinerea at the time – if we have a value of 100%, it means that there has been several times a wetness period long enough to infect the susceptible tissue. We calculate so-called “wet points” between leaf wetness and temperature with a maximum of initially 38400 points (beginning of the season, which displays 30% risk). After this period, each wet period with about 4000 wet points increases the risk by 10%, or on the other side, each dry period reduces the risk by ⅕ of the former value.
Literature
- Broome, J. C., English, J. T., Marois, J. J., Latorre, B. A., & Aviles, J. C. (1995). Development of an infection model for Botrytis bunch rot of grapes based on wetness duration and temperature. Phytopathology, 85(1), 97-102.
- Elmer, P. A., & Michailides, T. J. (2007). Epidemiology of Botrytis cinerea in orchard and vine crops. In Botrytis: biology, pathology and control (pp. 243-272). Dordrecht: Springer Netherlands.
- Williamson, B., Tudzynski, B., Tudzynski, P., & Van Kan, J. A. (2007). Botrytis cinerea: the cause of grey mould disease. Molecular plant pathology, 8(5), 561-580.
- Ciliberti, N., Fermaud, M., Roudet, J., & Rossi, V. (2015). Environmental conditions affect Botrytis cinerea infection of mature grape berries more than the strain or transposon genotype. Phytopathology, 105(8), 1090-1096.
Anthracnose
Pathogen
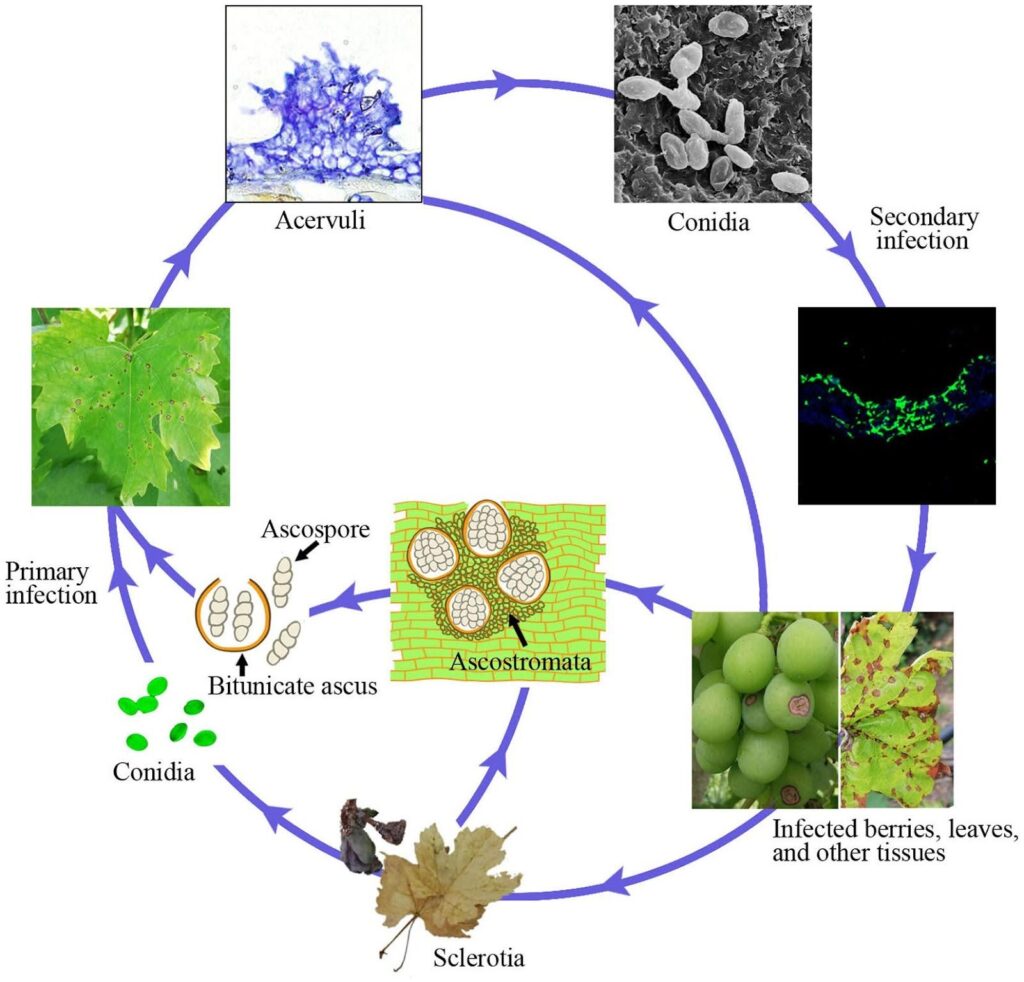
Anthracnose in grapevine is caused by the pathogen Elsinoe ampelina.
Infected canes are the main source of the disease. Sclerotia and mycelia surviving in the lesions and berries during winter become active in spring and produce ascospores (sexual spores) and conidia (asexual spores) under wet conditions (rain or dew for 24 hours) within the temperature range of 2~40°C.
These spores are dispersed to new tissues by rain splash and wind and once landing, they germinate and form germ tubes and appressoria, initiating new infection. They can infect new leaves, shoots, tendrils, and young berries. Warm weather reduces the wet duration required for the initial infection and incubation period. Optimum spore germination occurs at 25~30°C, with a minimum of 3~4 hours of leaf wetness required. At infections at temperature around 10°C, the incubation period is about 14 days.
As colonization progresses, acervuli emerge and new conidia are produced, which serve as secondary infection inoculum. They’re responsible for rapid and further infection in the season.
Symptoms
E. ampelina attacks the aerial, succulent parts of the vine, including shoots, leaves, petioles, tendrils, rachises, and berries; lesions on shoots and berries are most common. Grapes have ontogenic resistance to the fungi so young tissues are more susceptible.
Small reddish brown spots appear at first and enlarge in wet weather, becoming slightly sunken with a grey center surrounded by a dark margin. Sometimes, symptoms may look like hail injury. The necrotic center on leaves usually drops out, creating a “shot-hole” appearance.
In severe infection, plants may exhibit early defoliation, stunt cane growth, stem breakage, berry drop, and delayed development and ripening of berries.
FieldClimate Model
Viticulture Anthracnose Model
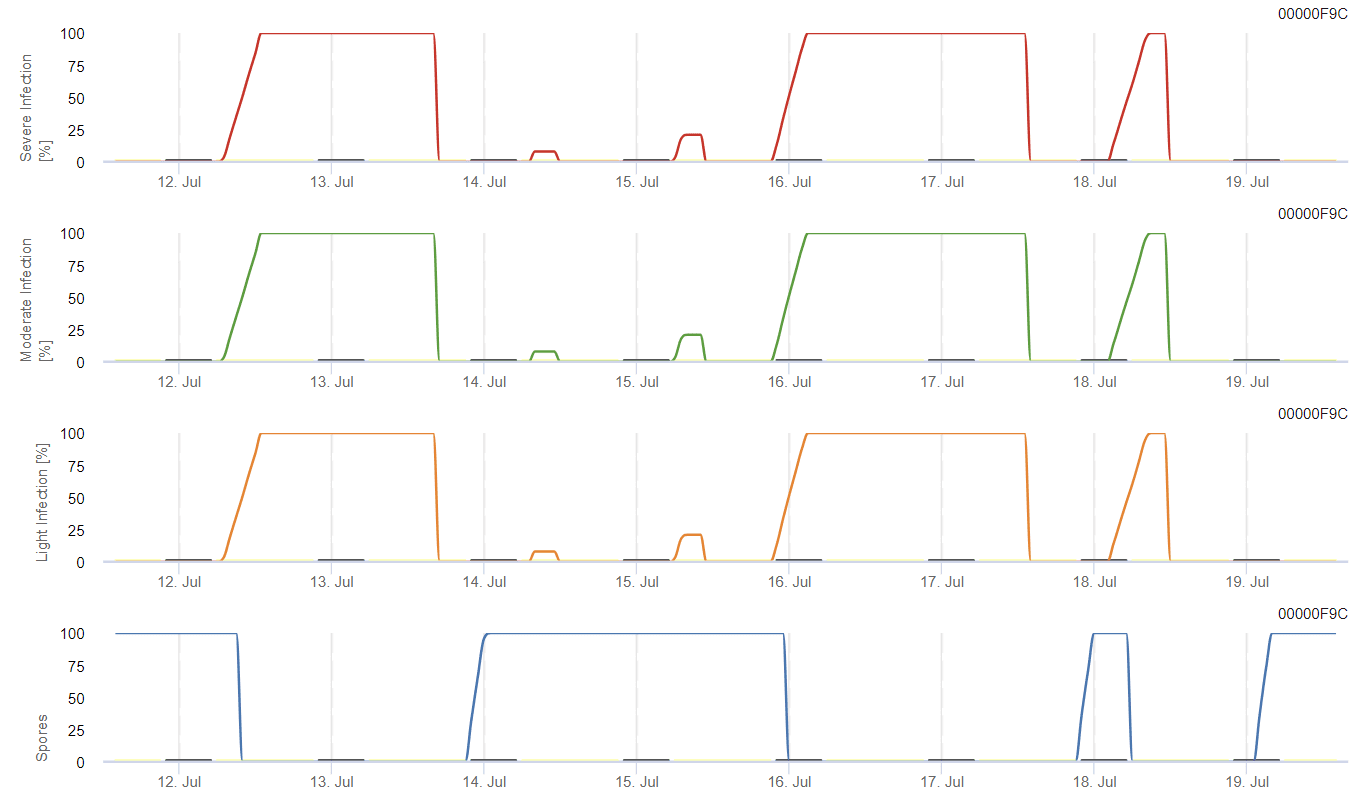
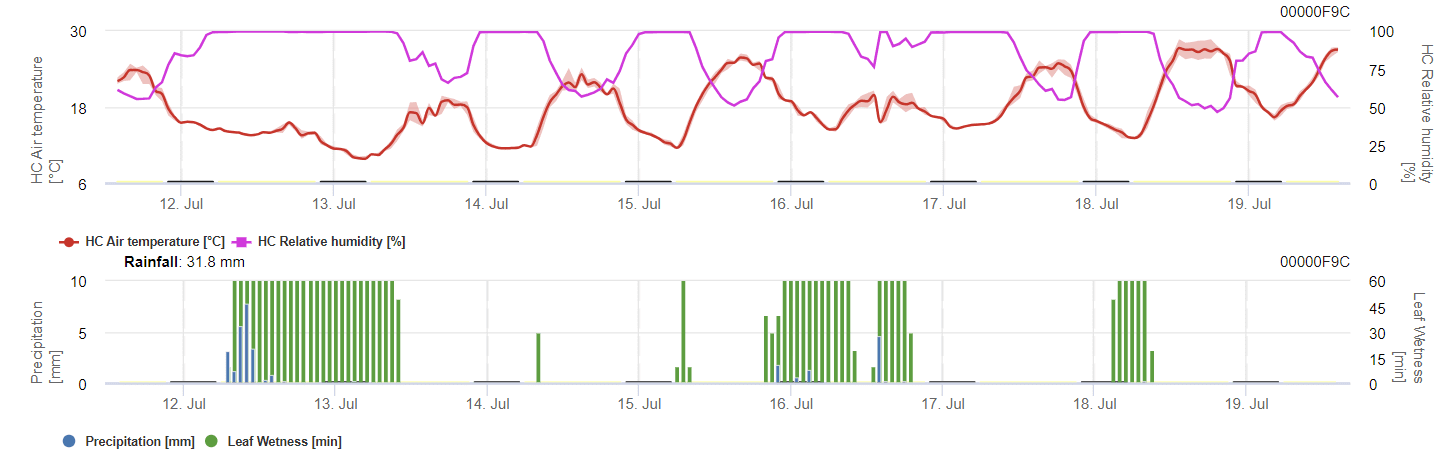
Sensors needed:
- Air temperature
- Relative humidity
- Leaf wetness
- Precipitation
The model calculates the risk of anthracnose by considering air temperature, relative humidity, leaf wetness, and precipitation.
The possible development of a weak, moderate, and severe infection are displayed in separate graphs. An infection has to be assumed to be complete when the increasing curve reaches 100%. The last graph shows the sporulation of E. ampelina; if the value there reaches 100%, it is assumed that spores of E. ampelina are present.
For overwintering spores to develop, conditions should meet a temperature range of 2 to 40°C, relative humidity above 90%, or leaf wetness. Once spore development reaches 100%, the infection starts to be calculated. The severity of the infection depends on wet conditions (rain events). However, if humidity drops below 50%, both spore development and infection stops to be calculated.
Literature
- Li, Z., Dos Santos, R. F., Gao, L., Chang, P., & Wang, X. (2021). Current status and future prospects of grapevine anthracnose caused by Elsinoe ampelina: An important disease in humid grape‐growing regions. Molecular Plant Pathology, 22(8), 899-910
PHOMOPSIS CANE AND LEAF SPOT
Pathogen
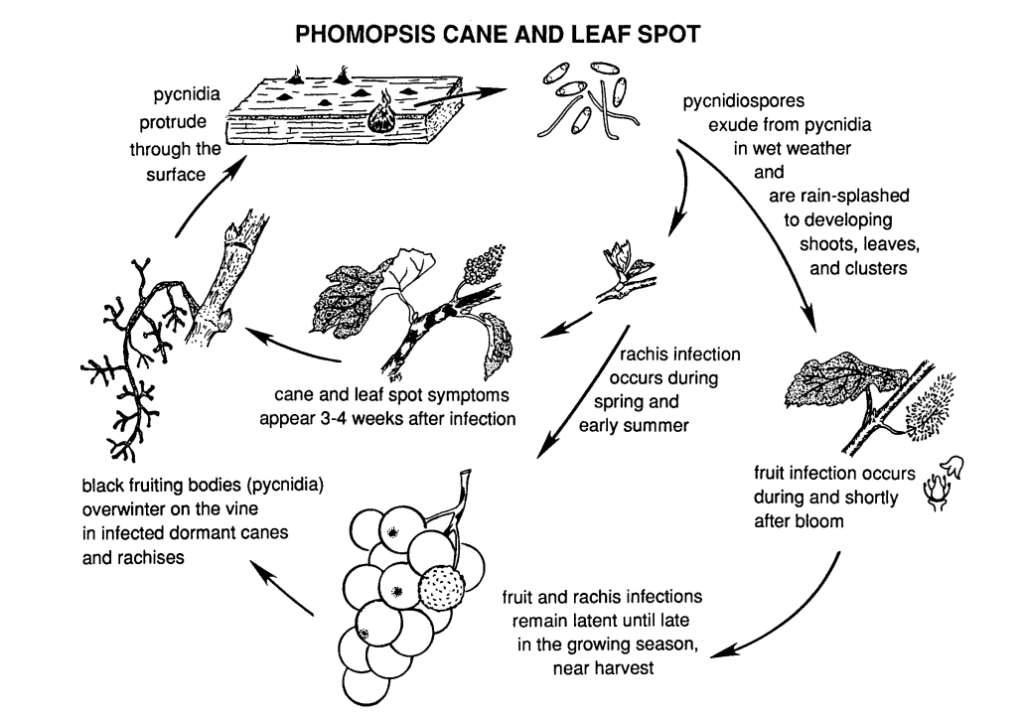
Phomopsis cane and leaf spot in grapevine is caused by Phomopsis viticola.
The fungus overwinters in the woody parts of the vine and becomes active again in the following spring. Once temperatures rise and spring rains begin, spores are released from overwintering structures called pycnidia. As spores are dispersed via rain and wind onto new tissues, infection spreads.
The primary infection period typically coincides with early spring rains, beginning shortly after bud break when shoots exhibit an early stage of growth. Young shoots, shoot tips, fruit clusters, and the rachis are all vulnerable to infection. Although shoot tips can become infected throughout the growing season, infections are most common from bud break to bloom. In fruits, the pathogen may remain dormant until the fruits ripen. The incubation period usually lasts about 3 to 4 weeks.
Symptoms
On leaves and petioles, small dark spots with black centers surrounded by yellow margins emerge, eventually coalescing. Basal leaves may become distorted, and crinkled, and may not develop to full size. When petioles are heavily infected, they yellow and fall off, leading to leaf drop. Leaves create an umbrella effect that reduces further infections from spores.
Similar spots appear on shoots, causing the epidermal layers to crack at the infection sites. As spots merge, the infected shoot areas develop a scabby texture. In severe cases, shoots may become stunted, break, or even die.
Lesions on the rachis cause it to become sunken and brittle. After a period of dormancy in summer, the fungus reactivates in early fall, leading to berry and bunch rot. While fruit infections are not extensive in general, spots do form on berries, often accompanied by black specks (pycnidia) on the berry skin. Under wet conditions, yellow spore masses may ooze from pycnidia, leading to fruit discoloration, rough texture, and mummification.
Infected wood shows a bleached appearance in the dormant season. Severely infected canes and spurs display dark discolorations mixed with bleached areas in the bark. Pycinidia may also break through the surface. Low temperatures, along with impaired phloem and xylem function, weaken and kill canes, spurs, and buds.
FieldClimate Model
Viticulture Phomopsis Infection Model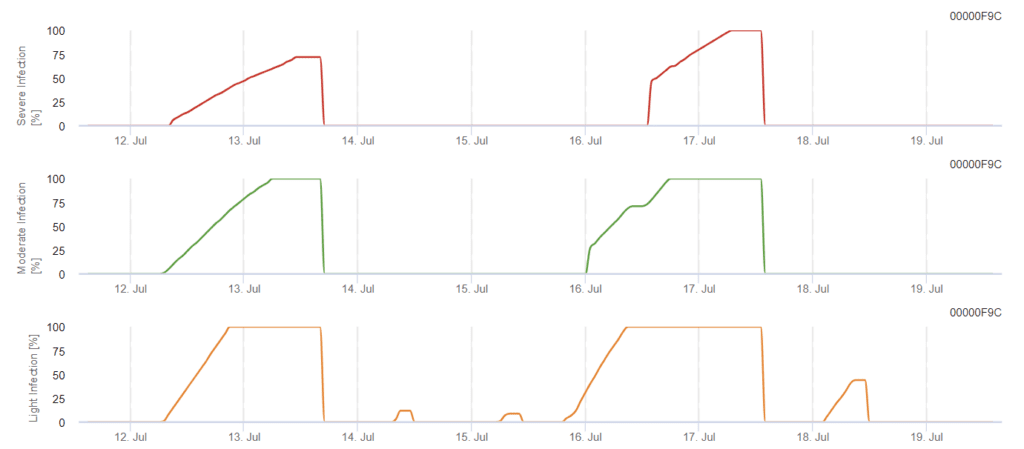
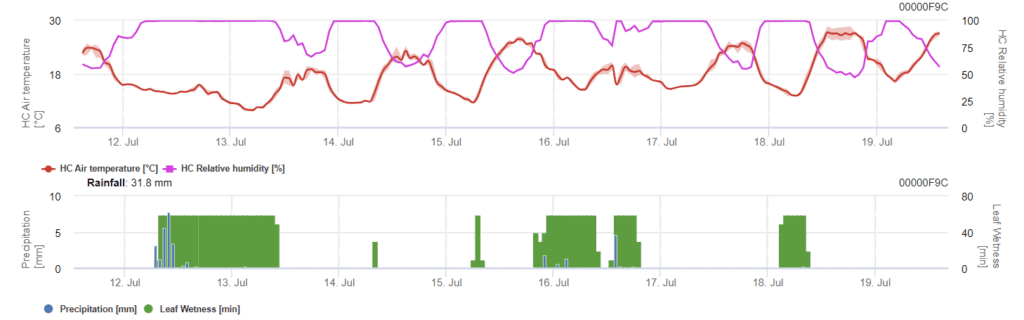
Sensors needed:
- Air temperature
- Relative humidity
- Leaf wetness
- Precipitation
The model determines the risk of Phomopsis infection at the temperature range of 5 to 35°C and wet conditions (leaf wetness, high relative humidity). The severity of the infection depends on the amount of rain (more than 2mm) as spores are distributed faster to healthy plant material. When 100% infection is shown, it indicates that optimal conditions have been measured in the field to infect plant tissue and thus, plant protection measurements should be taken into account.
Literature
- https://agriculture.vic.gov.au/biosecurity/plant-diseases/grapevine-diseases/phomopsis-cane-and-leaf-spot-of-grapevines
- Bettiga, L. J. (Ed.). (2013). Grape pest management (Vol. 3343). UCANR Publications.
- Pscheidt, J. W., & Pearson, R. C. (1991). Phomopsis cane & leaf spot.
Recommended equipment
Check which sensor set is needed for monitoring this crop’s potential diseases.
