
Tomato disease models
Alternaria
Pathogen
Alternaria species, notably Alternaria solani and Alternaria alternata, are fungal pathogens responsible for diseases like early blight and leaf spot in tomato plants. These fungi primarily overwinter in infected plant debris, seeds, and soil, surviving as spores or mycelium. In the spring, under favorable conditions, they produce conidia (asexual spores) that disperse through wind, water splashes, or contaminated equipment, initiating new infections. The pathogens can infect tomato plants at any growth stage, with initial symptoms often appearing on older, lower leaves.
Infection and disease development are favored by warm, moist, and humid conditions. Optimal temperatures for Alternaria species range between 15°C and 27°C, coupled with high relative humidity and extended periods of leaf wetness. These environmental conditions promote spore germination and rapid disease progression, especially during prolonged periods of wet weather. The pathogens can infect tomato plants at any growth stage, with initial symptoms often appearing on older, lower leaves.
Symptoms
The initial symptoms of Alternaria infections manifest as small, dark, circular lesions on the older, lower leaves of the tomato plant. These spots often develop characteristic concentric rings, giving them a “bullseye” appearance. As the disease progresses, lesions may enlarge, coalesce, and cause the affected leaves to yellow, wither, and eventually die, leading to significant defoliation. While the fungus primarily infects foliage, it can also affect stems and fruits. Stem lesions appear as dark, sunken areas, which can girdle the stem and impede nutrient flow, leading to plant wilting and death. Fruit infections typically occur at the stem end, presenting as sunken, black lesions that can render the fruit unmarketable. Severe defoliation due to Alternaria infections weakens the plant, reduces photosynthetic capacity, and exposes fruits to direct sunlight, increasing the risk of sunscald. This not only diminishes fruit quality but also reduces overall yield.
FieldClimate Model
Required Sensors:
- Air Temperature
- Relative Humidity
- Leaf Wetness
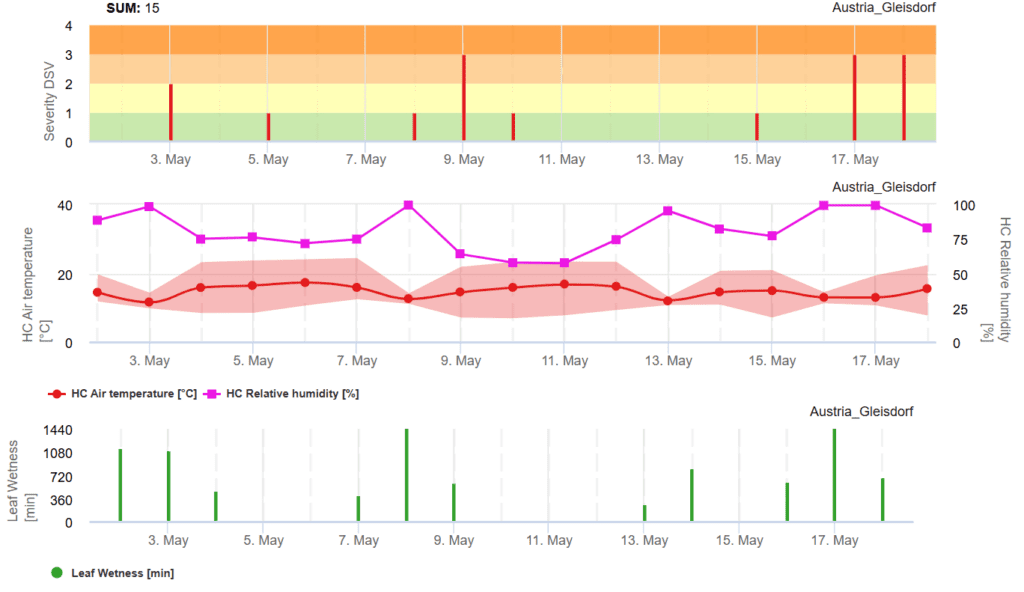
TomCast (TOMato disease foreCASTing) is a computer model that uses field data to predict fungal disease development in tomato crops. The model analyzes weather conditions from the past 24 hours to determine a Disease Severity Value (DSV)—a measure of disease progression. As DSVs accumulate, disease pressure on the crop increases.
When the total accumulated DSVs exceed the recommended spray threshold, a fungicide application is advised to reduce disease risk. The graph displays both individual DSVs and their cumulative sum. The recommended spray interval typically falls between 15 and 20 DSVs. Once the total accumulated DSVs surpass this threshold, a fungicide spray is necessary to protect the crop.
Literature:
Gillespie, T. J., Srivastava, B., & Pitblado, R. E. (1993). Using operational weather data to schedule fungicide sprays on tomatoes in southern Ontario, Canada. Journal of Applied Meteorology and Climatology, 32(3), 567-573.
Grey mould
Pathogen
Gray mold (Botrytis cinerea) overwinters as sclerotia or mycelium in plant debris and can sometimes be seedborne as spores or mycelium in certain crops. Other plant species may also serve as reservoirs for the pathogen, increasing the likelihood of cross-infection. The airborne conidia can be dispersed by wind and may also be carried on the surface of splashing rain droplets. High relative humidity is essential for prolific spore production. In the field, spores that land on tomato plants germinate and initiate infection when free water from rain, dew, fog, or irrigation is present on the plant surface.
The optimal temperature range for infection is between 18°C and 24°C, with infection occurring within as little as five hours under favorable conditions. However, high temperatures above 28°C inhibit fungal growth and spore production. Dying flowers provide an ideal site for infection, but the pathogen can also infect plants through direct contact with moist, infested soil or plant debris. In greenhouse environments, stem lesions develop either from direct colonization of wounds or through infected leaves. The presence of external nutrients, such as pollen grains in water droplets, can significantly enhance infection. Additionally, the type of wound influences stem lesion development—breaking off leaves results in a lower incidence of stem lesions compared to cutting leaves with a knife, which leaves a stub behind.
Symptoms
Symptoms of B. cinerea infection in tomatoes vary depending on the affected plant part. On leaves, initial signs include water-soaked, beige lesions that may expand and coalesce, leading to necrosis.
Infected stems can develop brownish lesions, potentially girdling the stem and causing wilting or plant death. Flowers are also susceptible, with infections often leading to fruit rot. Infected fruits exhibit soft, watery decay, often accompanied by a grayish, fuzzy mold on the surface. This mold consists of conidiophores and conidia, which contribute to further disease spread. Under humid conditions, the pathogen can rapidly colonize and destroy fruit tissues, leading to significant yield losses. Recognizing these symptoms is crucial for early detection and management of gray mold in tomato crops.
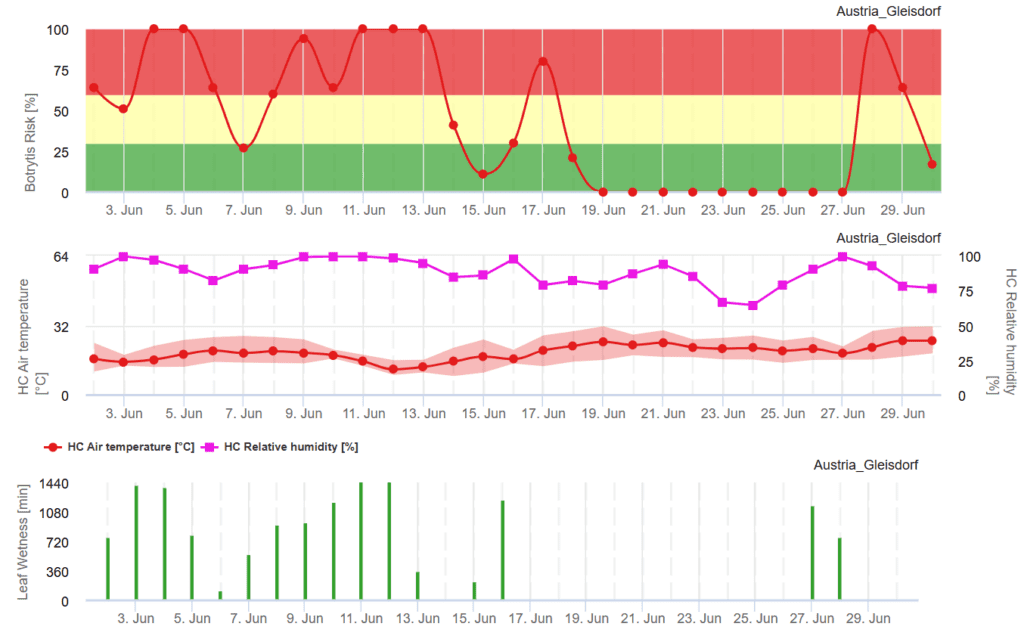
FieldClimate Model
Sensors needed:
- Air temperature
- Leaf wetness
- Solar radiation
Literature:
Jacob, D., David, D. R., Sztjenberg, A., & Elad, Y. (2008). Conditions for development of powdery mildew of tomato caused by Oidium neolycopersici. Phytopathology, 98(3), 270-281.
Jacob, D., David, D. R., & Elad, Y. (2007). Biology and biological control of tomato powdery mildew (Oidium neolycopersici). IOBC WPRS BULLETIN, 30(6/2), 329.
Fletcher, J. T., Smewin, B. J., & Cook, R. T. A. (1988). Tomato powdery mildew. Plant Pathology, 37(4), 594-598.
Jones, H., Whipps, J. M., & Gurr, S. J. (2001). The tomato powdery mildew fungus Oidium neolycopersici. Molecular Plant Pathology, 2(6).
Cladosporium fulvum
Pathogen
Cladosporium fulvum, also known as Passalora fulva, is a fungal pathogen responsible for tomato leaf mold, predominantly affecting tomato plants in greenhouse environments. The pathogen overwinters in plant debris, seeds, and soil as sclerotia or conidia, which can survive without a host for at least one year. When favorable conditions arise, these survival structures produce new conidia that serve as primary inoculum. The conidia germinate on the leaf surface, penetrating the plant through stomata, especially under high humidity conditions. Subsequently, conidiophores develop on the underside of infected leaves, leading to the production of more conidia that disperse via wind, water splashes, tools, clothing, and insects, facilitating rapid disease spread.
Infection and disease development are favored by high humidity and moderate temperatures. Relative humidity levels of 75 to 90% are needed, with an optimum RH of 85%. The disease develops at temperatures between 40° and 90°F (4° and 32°C), with the optimum between 72° and 76°F (22° and 24°C). The disease rarely occurs at temperatures below 50°F (10°C). New spores can form within ten to twelve days after infection, and there can be multiple cycles per season.
Symptoms
The initial symptoms of tomato leaf mold appear on older leaves as pale green or yellowish spots with indefinite margins on the upper surface. Correspondingly, on the lower leaf surface, the fungus sporulates, producing olive-green to grayish-purple, velvety patches composed of spores. As the disease progresses, infected areas may become necrotic, causing the leaves to curl, wither, and eventually die, often remaining attached to the plant.
While the disease primarily impacts tomato leaves, severe cases may affect tomato stems, flowers, and fruits. Infected blossoms may turn black and fall off, leading to reduced fruit set. Fruit symptoms are black, irregularly shaped lesions with a diffuse edge on green or mature fruit. Crop yield is reduced significantly when more than 50% of the leaf area is covered by lesions.
FieldClimate Model
Required sensors:
- Air Temperature
- Relative Humidity
- Leaf Wetness
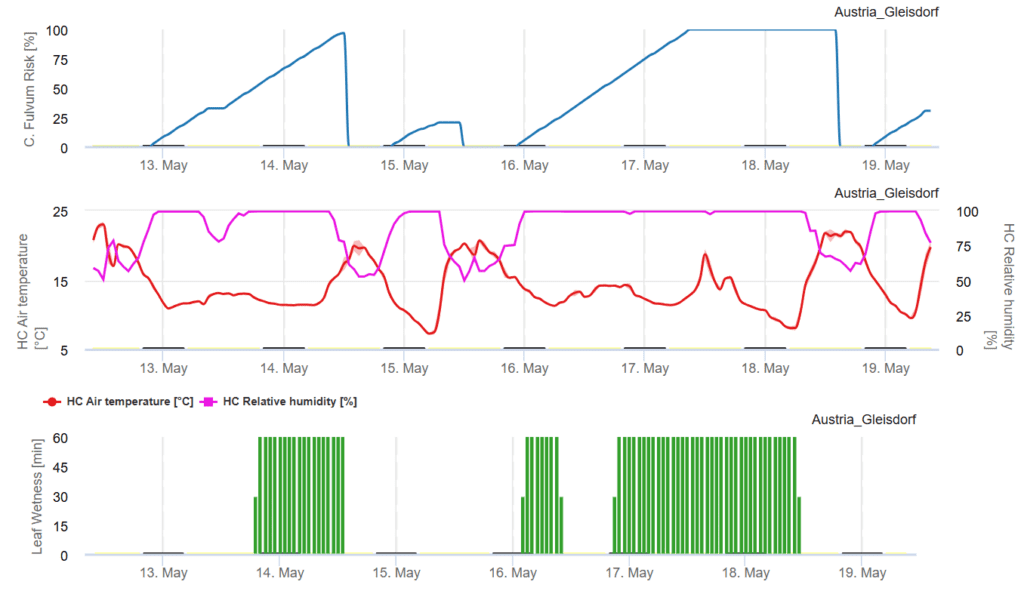
We assess the risk of a Cladosporium fulvum infection by monitoring leaf wetness, relative humidity, and air temperature. The graph highlights periods of high infection risk when the curve reaches between 80% and 100%.
Literature:
Agriculture and Horticulture Development Board. (n.d.). Tomato leaf mould: Cause, symptoms & spread. AHDB. Retrieved March 10, 2025, from https://horticulture.ahdb.org.uk/knowledge-library/cause-symptoms-spread-tomato-leaf-mould
Colletotrichum spp
Pathogen
Colletotrichum species are pathogenic fungi responsible for anthracnose in tomatoes. The pathogen survives unfavorable conditions by forming resilient structures called sclerotia, which persist in soil and plant debris. As temperatures rise in late spring, these sclerotia germinate, releasing spores that infect the lower leaves and fruits of tomato plants. Initial infections often occur on senescent leaves or those damaged by pests, serving as primary sources for secondary infections throughout the growing season.
The development and spread of Co are significantly influenced by environmental conditions. The fungus thrives in warm temperatures, with optimal growth around 80°F (approximately 27°C), but it remains active between 55°F and 95°F (13°C to 35°C). Wet weather, especially prolonged periods of leaf wetness due to rain or overhead irrigation, promotes disease development. Splashing water facilitates the dispersal of spores from infected plant parts to healthy tissues, leading to new infection sites.
Symptoms
Symptoms of anthracnose caused by Colletotrichum spp. primarily manifest on tomato fruits, although stems, leaves, and roots can also be affected. On fruits, the disease presents as small, sunken, dark lesions that may enlarge over time, leading to extensive rotting. Both green and ripe fruits are susceptible; however, lesions on green fruits might not be immediately visible but develop as the fruit matures. Infected stems and leaves may exhibit dark spots or lesions, potentially compromising the plant’s overall health and vigor.
In addition to visible symptoms, Colletotrichum spp. can cause internal discoloration and decay, reducing the marketability and quality of tomato produce. Infected plants may experience premature leaf drop, reduced yield, and increased vulnerability to other pathogens due to the compromised integrity of plant tissues. Recognizing these symptoms early is crucial for implementing effective management strategies to mitigate the impact of anthracnose on tomato crops.
FieldClimate Model
Sensors needed:
- Air temperature
- Leaf wetness
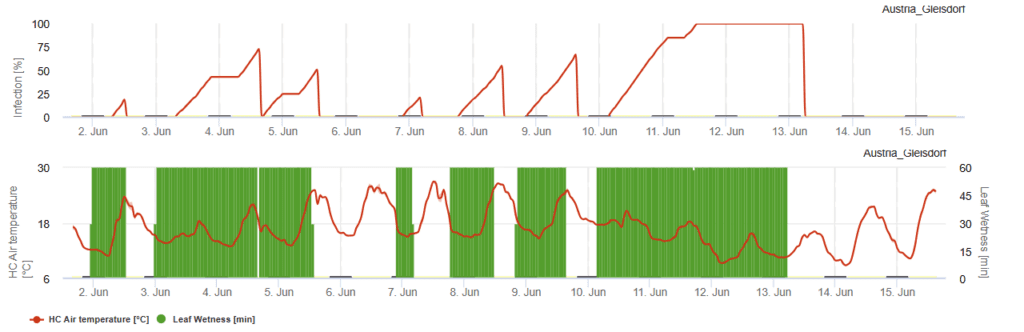
Anthracnose infects plants at temperatures ranging from 15°C to 30°C, but a long lasting leaf wetness period is needed. At optimum temperatures of 20°C to 25°C a leaf wetness period of 12 hours is needed. So, whenever the infection curve reaches 100% optimal conditions have been given in the field for an infection.
Literature:
Lees, A. K.; Hilton, A. J. (2003). Black dot (Colletotrichum coccodes): An increasingly important disease of potato. Plant Pathology, 52. Wiley-Blackwell: 3–12. doi:10.1046/j.1365-3059.2003.00793.x.
British Society for Plant Pathology (BSPP). Tomato | Diseases and Pests, Description, Uses, Propagation. Archived from the original on 2014-12-13. Retrieved 2014-12-22.
Phytophthora
Pathogen
Phytophthora species, notably Phytophthora infestans and Phytophthora capsici, are notorious pathogens affecting tomato plants. These oomycetes produce sporangia, which release motile zoospores capable of infecting host tissues. P. infestans thrives in moist, cool environments, with sporulation optimal at 12–18 °C (54–64 °F) and lesion growth rates peaking between 20–24 °C (68–75 °F). Similarly, P. nicotianae flourishes in warm temperatures ranging from 84–90 °F, requiring water-saturated conditions for spore dissemination.
The life cycle of these pathogens is heavily influenced by environmental conditions. Saturated soils and prolonged leaf wetness facilitate the movement and infection capabilities of zoospores. For instance, P. capsici causes damping-off in seedlings and thrives in wet, waterlogged soils, leading to rapid disease progression.
Symptoms
Symptoms of Phytophthora infections in tomatoes vary depending on the species involved. P. infestans causes late blight, characterized by water-soaked lesions on leaves, stems, and fruits, which rapidly enlarge and turn dark brown or black, often accompanied by a white, fuzzy growth under moist conditions. P. capsici leads to crown infections, leaf spots, and foliar blight, with fruit rot displaying concentric rings.
FieldClimate Models
Blight Phytophthora Capsici
Sensors needed:
- Air Temperature
- Relative humidity
We calculate the formation and infection potential of Phytophthora capsici through both oospores (sexual reproduction) and sporangia (asexual reproduction). Oospores have thick walls, allowing them to survive for long periods, but their formation requires the presence of two compatible P. capsici isolates. In contrast, sporangia are rapidly dispersed within the crop, facilitating quick infection. When infection reaches 100%, it indicates that optimal conditions for the development and spread of either oospores or sporangia have been met.
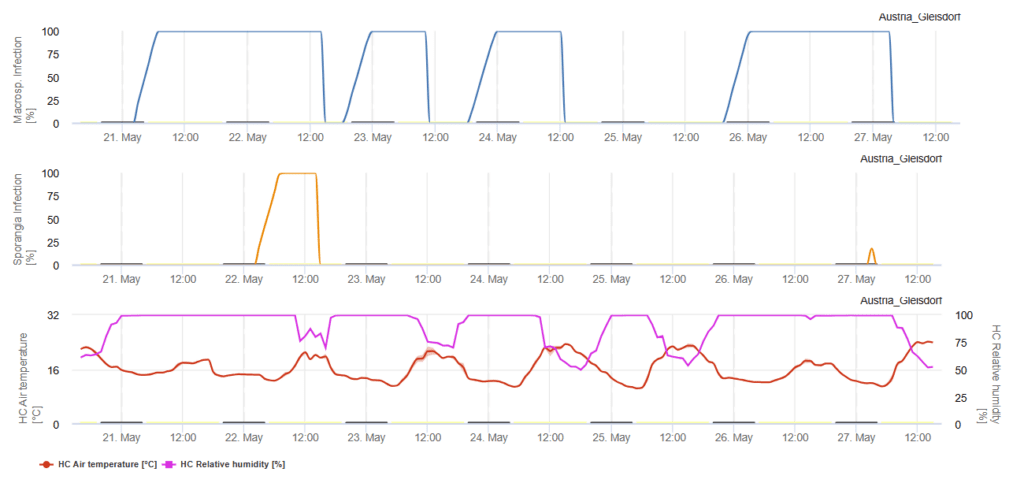
Phythopthora infestans model NoBlight
Sensors needed:
- Air Temperature
- Relative Humidity
- Leaf Wetness
- Precipitation
The likelihood of Late Blight occurring is predicted using accumulated “Severity Values,” which are based on weather conditions, particularly mild and wet environments that favor pathogen development. These values increase when conditions are suitable for disease progression. We display the Severity Values alongside the recommended spray intervals, with shorter intervals when severity is high and longer intervals when severity is low.
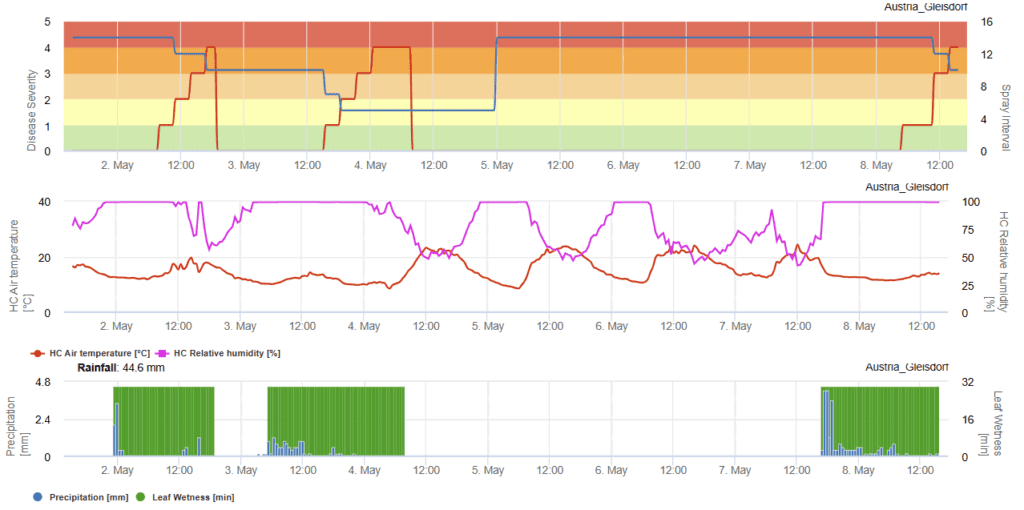
Phytophthora infestans model Fry
Sensors needed:
- Air Temperature
- Relative Humidity
- Leaf Wetness
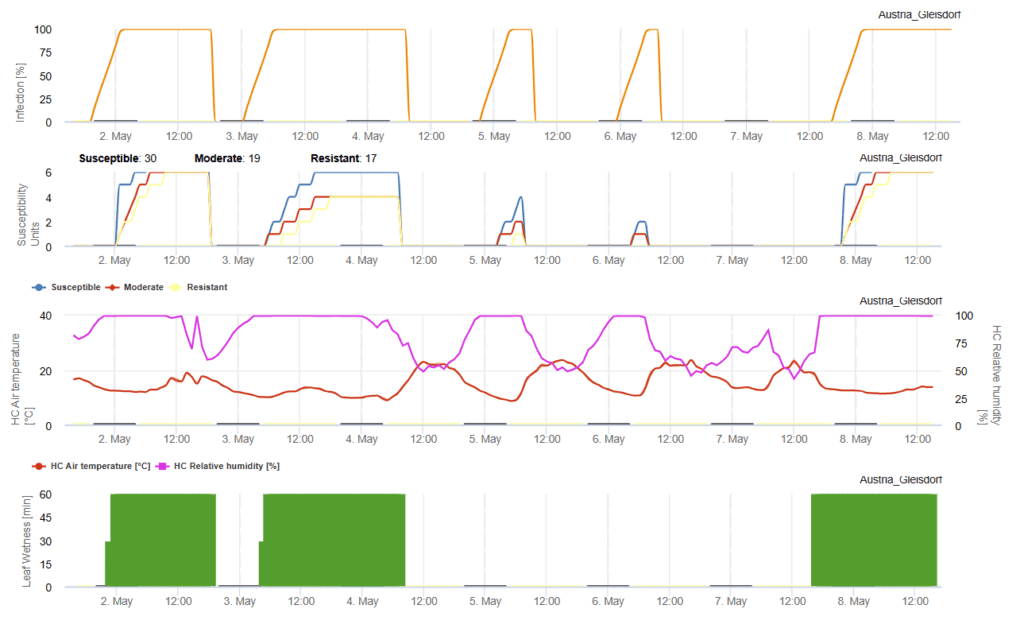
W.E. Fry (1983) studied potato infection under different susceptibility levels, examining the effects of high humidity, leaf wetness, and temperature. Based on his findings, he developed an infection model for late blight in potatoes and later expanded it to estimate optimal spray intervals for the fungicide chlorothalonil (Bravo). His research showed that susceptible cultivars are infected more quickly and with greater severity, while moderately susceptible and resistant varieties require longer moisture exposure or warmer temperatures for infection, resulting in lower disease severity.
The model assigns infection period ratings, with a maximum of 7 for susceptible varieties, 6 for moderately susceptible, and 5 for resistant ones. It also determines fungicide application timing based on accumulated blight units, which must not exceed 30 for susceptible, 35 for moderately susceptible, and 40 for moderately resistant varieties. A new spray is needed if more than six days have passed since the last application and the blight unit threshold is surpassed. This model is an effective tool for guiding fungicide use to manage late blight in potatoes.
Literature:
Fry, WE, AE Apple & JA Bruhn (1983). Evaluation of potato late blight forecasts modified to incorporate host resistance and fungicide weathering. Phytopathology 73:1054-1059.
Powdery Mildew
Pathogen
Powdery mildew in tomatoes may be caused by three pathogens worldwide: Leveillula taurica, Erysiphe orontii, and Oidium lycopersicum.
Leveillula taurica has various host species in warm arid to semi-warm arid climates in Asia, the Mediterranean, Africa, and more recently in the southwest United States. Erysiphe orontii is another common species with a wide range of host plants in both temperate and tropical regions. The third pathogen, Oidium lycopersicum, differs from others as no sexual stage has been identified for this species. Besides, Oidium neolycopersici has been classified separately from Oidium lycopersici, exhibiting different molecular and morphological characteristics and is a critical threat to greenhouse and field-grown tomatoes.
Conidia (asexual spores) are produced from conidiophores and dispersed by wind. Once landing on a new host, they germinate and produce germ tubes, followed by the formation of appressorium and haustorium. These structures allow the fungus to penetrate and extract nutrients from the host. Rapid and continuous colonization occurs as secondary hyphae spread across and the asexual cycle repeats as new conidiophores are produced.
The sexual stage begins with the formation of cleistothecia, which serves as overwintering structures. Under favorable environmental conditions, these cleistothecia release ascospores (asexual spores), and these spores land on a new host, initiating new infection through a similar process as conidia.
Symptoms
White powdery lesions appear on the upper surfaces and occasionally on the lower surfaces of the leaves. They also develop on other parts of the plant like petioles, stems, and sepals but not on fruits – L. taurica causes symptoms only on leaves. In severe cases, lesions may coalesce, covering the entire surface. Although fruits are not directly affected, impaired photosynthesis and premature senescence may impact fruit size and nutritional quality, resulting in yield loss.
FieldClimate Model
There are three models for different types of tomatoes.
- Covered Tomato: General Tomato Powdery Mildew Risk Model, General Tomato Powdery Mildew Risk PE Model, Leveillula Taurica Powdery Mildew Model
- Modered Climate Tomato: General Tomato Powdery Mildew Risk Model
- Warm Climate Tomato: General Tomato Powdery Mildew Risk Model, General Tomato Powdery Mildew Risk PE Model, Leveillula Taurica Powdery Mildew Model
Here are some environmental conditions favoring the infection:
- relative humidity levels > 50% (optimum RH > 90%)
- free water on leaf surfaces is not necessary
- temperature range: 10-35 °C (best below 30 °C)
General Tomato Powdery Mildew Risk Model
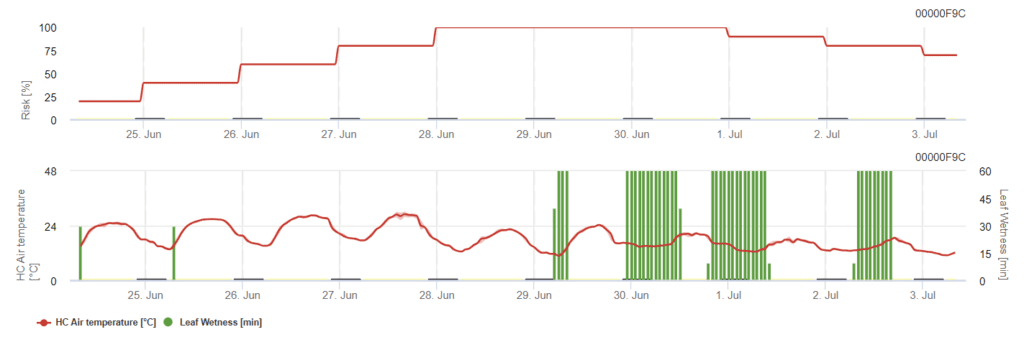
Sensors needed:
- Air temperature
- Leaf wetness
Powdery mildew is an inoculum-driven disease, therefore just risky periods are determined. This model calculates the risk based on leaf wetness and air temperature. When the risk index exceeds 80%, control strategies should be considered: combine the modeling of the risky period with the fungal inoculum monitoring.
General Tomato Powdery Mildew Risk PE Model
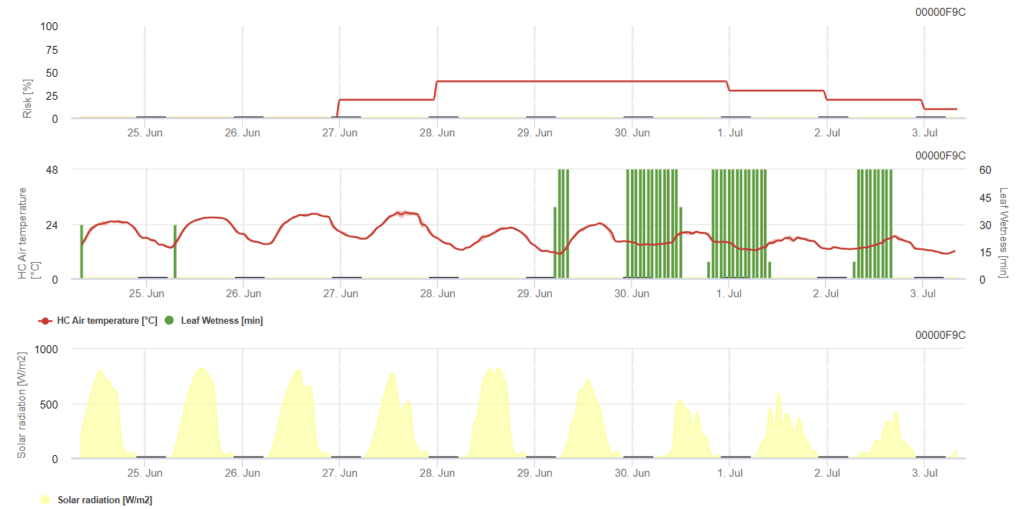
Sensors needed:
- Air temperature
- Leaf wetness
- Solar radiation
This model is essentially the same as the previous one, with the key difference of including solar radiation. When the risk index exceeds 80%, control strategies should be considered: combine the modeling of the risky period with the fungal inoculum monitoring.
Leveillula Taurica Powdery Mildew Model
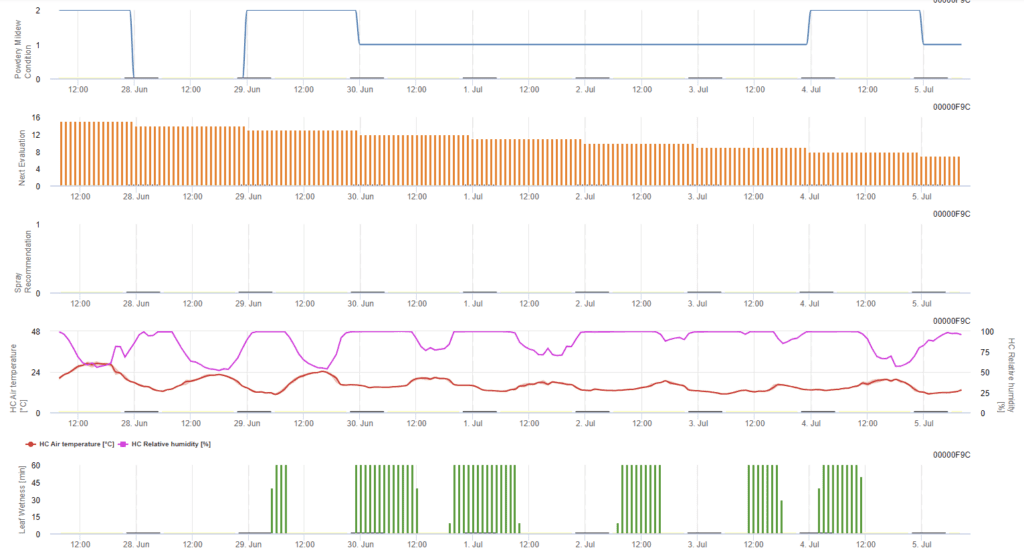
Sensors needed:
- Air temperature (avg, min, max)
- Relative humidity
- Leaf wetness
This model is based on the forecast model developed by the University of California, Davis. Favorable conditions for powdery mildew are determined based on temperature, relative humidity, and leaf wetness and are depicted on the first graph. Based on this, the next evaluation of the disease and the spray recommendation are given.
Literature:
Jacob, D., David, D. R., Sztjenberg, A., & Elad, Y. (2008). Conditions for development of powdery mildew of tomato caused by Oidium neolycopersici. Phytopathology, 98(3), 270-281.
Jacob, D., David, D. R., & Elad, Y. (2007). Biology and biological control of tomato powdery mildew (Oidium neolycopersici). IOBC WPRS BULLETIN, 30(6/2), 329.
Fletcher, J. T., Smewin, B. J., & Cook, R. T. A. (1988). Tomato powdery mildew. Plant Pathology, 37(4), 594-598.
Jones, H., Whipps, J. M., & Gurr, S. J. (2001). The tomato powdery mildew fungus Oidium neolycopersici. Molecular Plant Pathology, 2(6).
Septoria leaf spot
Pathogen
Septoria lycopersici is a fungal pathogen responsible for Septoria leaf spot, a common and destructive disease affecting tomato plants worldwide. The fungus primarily overwinters on infected plant debris, such as fallen leaves and stems, as well as on certain solanaceous weeds like nightshade. In the spring, under favorable conditions, the fungus produces spores that are dispersed by wind and splashing rain, leading to new infections. The pathogen can infect tomato plants at any stage of development, with symptoms typically appearing on the lower leaves after the first fruit sets.
Infection and disease development are favored by warm, wet, and humid conditions. Optimal temperatures for the spread of S. lycopersici range between 60°F and 80°F (15°C to 27°C), coupled with high relative humidity and extended periods of leaf wetness. These environmental conditions promote the release and dispersal of spores, which can lead to rapid disease progression, especially during prolonged periods of wet weather.
Symptoms
The initial symptoms of Septoria leaf spot manifest as small, circular lesions, approximately 1/16 to 1/4 inch in diameter, on the older, lower leaves of the tomato plant. These spots have dark brown margins with tan or gray centers and often contain tiny black fruiting structures known as pycnidia, which are diagnostic of the disease. As the lesions become more numerous, affected leaves may turn yellow, then brown, and eventually wither and die, leading to significant defoliation. While the fungus primarily infects foliage, it can occasionally affect stems and, rarely, the fruit. Severe defoliation due to Septoria leaf spot weakens the plant, reduces photosynthetic capacity, and exposes fruits to direct sunlight, increasing the risk of sunscald. This not only diminishes fruit quality but also reduces overall yield. Implementing integrated pest management strategies, such as crop rotation, removal of infected plant debris, and the use of fungicidal sprays, is essential to control the spread of S. lycopersici and minimize its impact on tomato crops
FieldClimate Model
Required sensors:
- Air Temperature
- Relative humidity
- Leaf Wetness
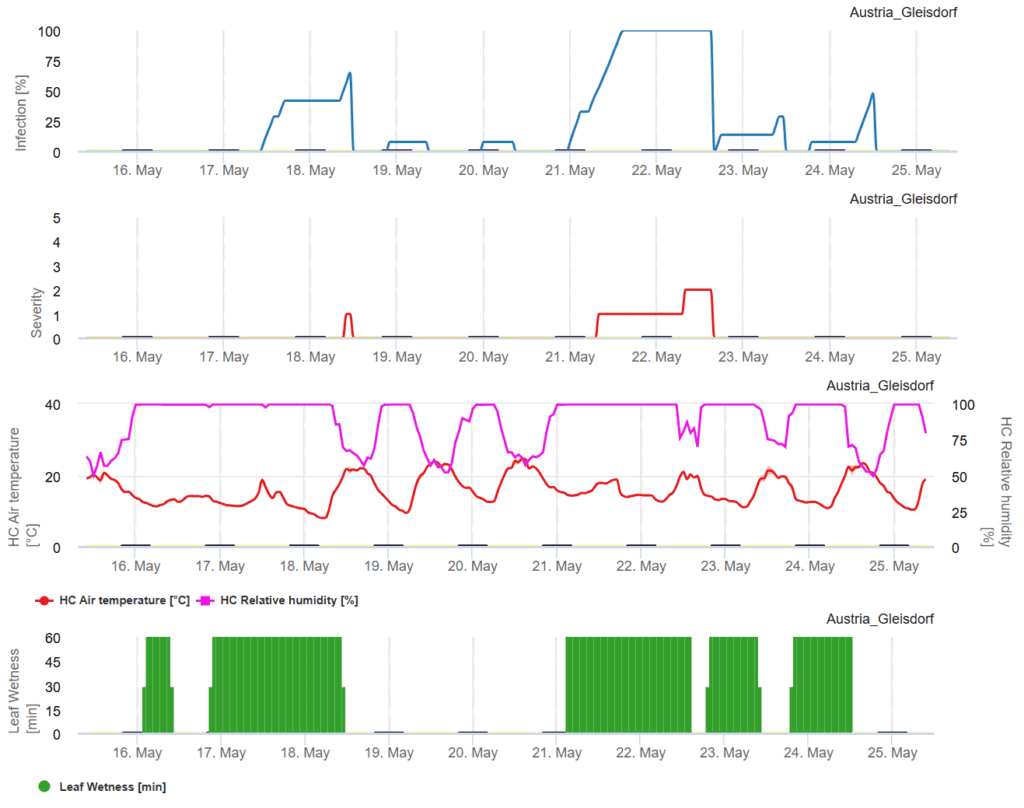
Wet conditions promote spore production when temperatures range between 15°C and 27°C. These spores can spread through wind, water, hands, insects, and equipment. Under moist conditions, spores may germinate within 48 hours, with leaf spots appearing in as little as five days, followed by new spore production. Prolonged dew and rainy weather further accelerate disease development. Increased infection risks are monitored using FieldClimate.com, where rising infection curves indicate favorable conditions. Once infection reaches 100%, curative plant protection measures should be implemented immediately.
Literature:
University of Illinois Extension. (n.d.). Septoria leaf spot of tomato, Septoria lycopersici.
Missouri Botanical Garden. (n.d.). Septoria leaf spot of tomato.
NC State Extension Publications. (n.d.). Septoria leaf spot of tomato.
Recommended equipment
Check which sensor set is needed for monitoring this crop’s potential diseases.
