Pulses disease models
Asochyta blight
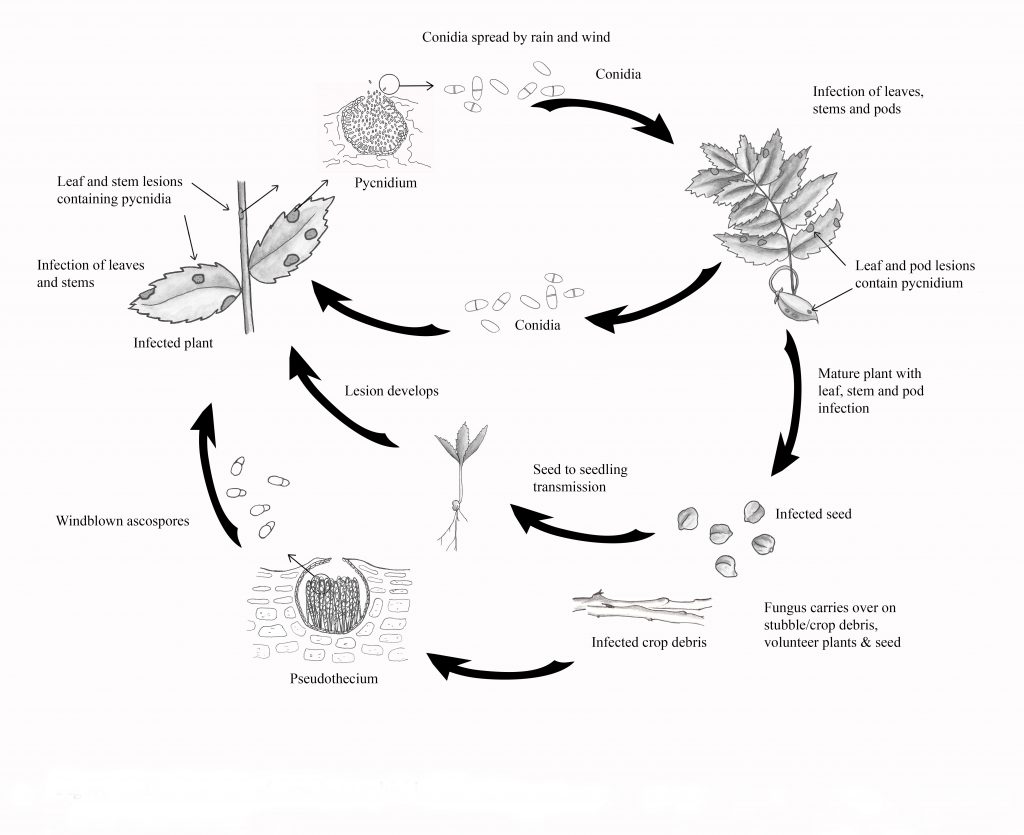
Life Cycle
The pathogen overwinters on infected crop residue and seed. Infected seed can play an important role in both the introduction of Ascochyta Blight into new areas, as well as in early disease development, as the pathogen is easily transmitted from the seed to seedlings. Both asexual spores (conidia, spread by rain-splash) and sexual spores (ascospores, spread by wind) can be produced on the crop residue. In the late fall and early spring, sexual reproduction produces pseudothecia which house the ascospores. Development of pseudothecia takes five to seven weeks under adequate moisture and moderate temperatures (near 10°C).
In spring and early summer, mature pseudothecia release ascospores into the air, which can travel for several miles to infect target crops. Airborne ascospores are thought to be the initial source of infection in the spring, although rain-splashed conidia may also be involved. After the spore infection takes place, symptoms begin to develop within four to six days. Early lesions are tan to dark brown, with a dark brown margin. Three to six days after lesions are formed, dark brown pycnidia develop.
Pycnidia are often arranged in concentric rings and will not rub off the tissue-like debris will. Conidia ooze out of the pycnidia in a sticky spore mass and are spread by rain-splash onto healthy plant parts, causing new infections.
The majority of the lesions during the growing season result from the rapid development of pycnidia and conidia under humid conditions. Even small rain showers are enough to spread conidia to new plant tissue. So it is called a polycyclic disease, meaning that multiple infection cycles can occur throughout the growing season under adequate moisture and temperatures (20 to 25°C).
Disease model
We model the development of pycnidia and conidia during growth season.
Ascochyta blight disease development is optimal at temperatures of 20 to 21°C (temperature range is 4°C to 34°C) and moist conditions (high relative humidity and leaf wetness).
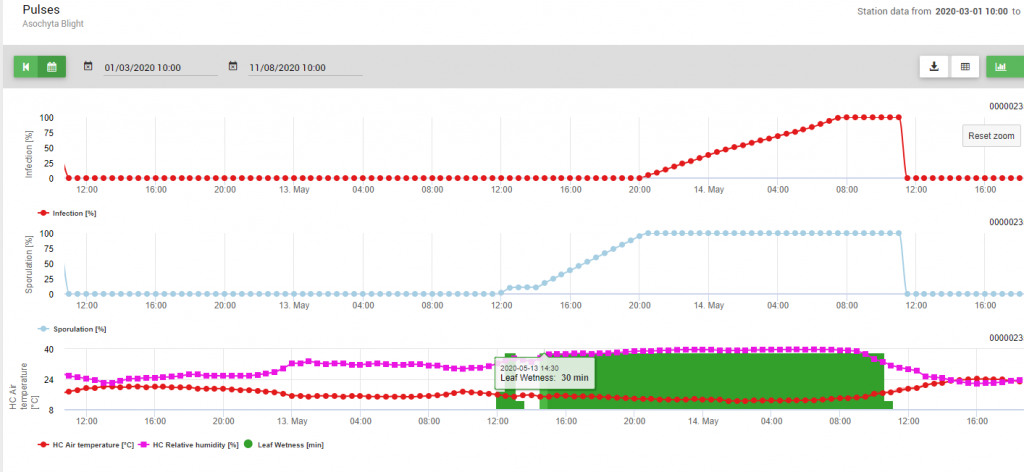
Implemented into the model are two steps of the disease cycle: 1. the Sporulation (development of pycnidia, the release of conidia), so producing of new infectious units and 2. optimal conditions for the further infection of those conidia. So for infection of new plant tissue first optimal conditions for the sporulation have been given (100%) and afterwards, the infection curve has reached 100%.
Because of optimal conditions (leaf wetness, high relative humidity and temperature around 15°C for a long time) the sporulation of pycnidia was determined on May 13th at 20:00 (blue line, 100%). Conditions were still optimal to start the infection progress (red line) and optimal conditions for infection have been determined on May 14th at 8: 00 in the morning (100% reached). So depending on the plant protection strategy prophylactic measurements should have been taken into account already before the infection took place or when using curative ones shortly after the infection (100%) was determined.
Literature:
Grey mould
Botrytis cinerea is a necrotrophic fungus that affects many plant species, although its most notable hosts may be grapes.
In viticulture, it is commonly known as botrytis bunch rot; in horticulture, it is usually called a grey mould or grey mould.
The fungus gives rise to two different kinds of infections on grapes. The first, grey rot, is the result of consistently wet or humid conditions, and typically results in the loss of the affected bunches. The second, noble rot, occurs when drier conditions follow wetter and can result in distinctive sweet dessert wines, such as Sauternes or the Aszú of Tokaj. The species name Botrytis cinerea is derived from the Latin for “grapes like ashes”; although poetic, the “grapes” refers to the bunching of the fungal spores on their conidiophores, and “ashes” just refers to the greyish colour of the spore masses. The fungus is usually referred to by its anamorph (asexual form) name because the sexual phase is rarely observed. The teleomorph (sexual form) is an ascomycete, Botryotinia cinerea.
Biology of B. cinerea
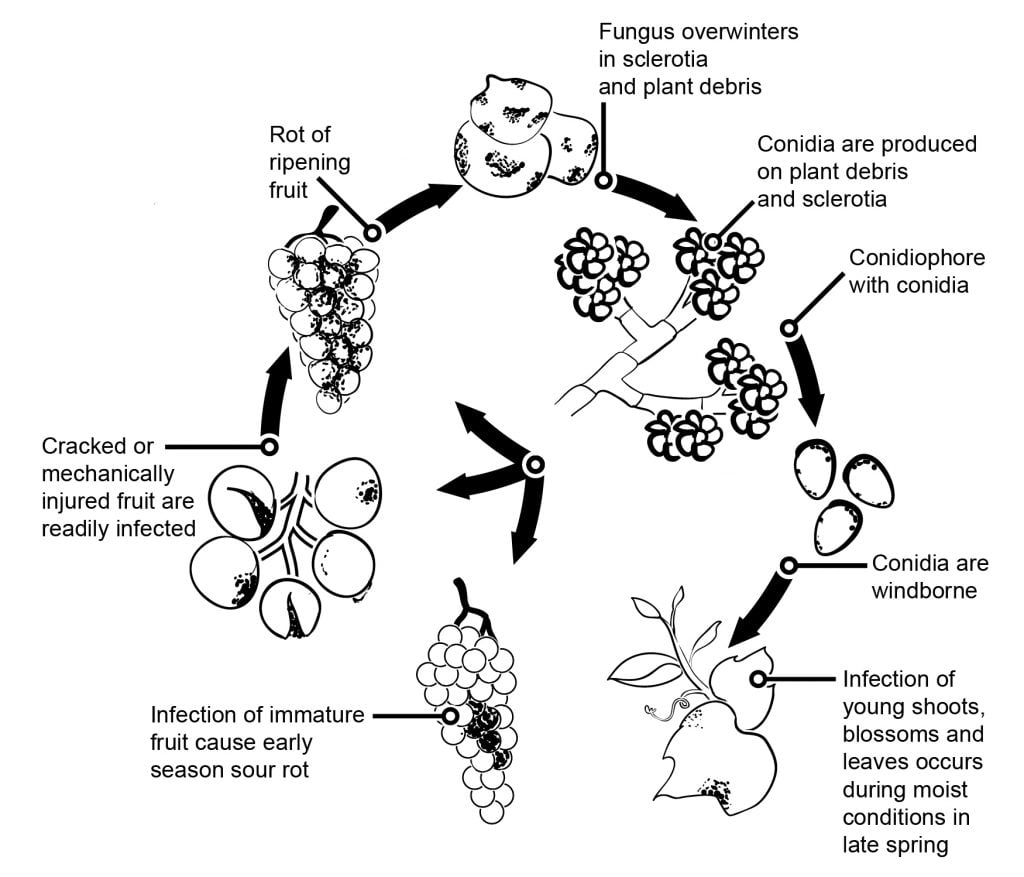
In fruits and grapes flower infections followed by latency are having a big impact on the epidemiology of grey mould. Several flowers to fruit infection pathways can be separated. In grapes, kiwifruits, raspberries infections trough the stylus into the ovule have been postulated. In the ovulus the pathogen stays latent, which seems to be a result of preformatted host defense strategy (similar to the resveratrol content of young grape berry). In grapes, kiwifruits and strawberries infections trough the stamen, petals or sepals have been found important. In grapes, studies showed that B. cinerea could infect the stamens and grows basipetal to infect the receptacle and then grows systemically to the pedicel and vascular tissues in the berries.

A 6-year research project in the Cape area showed that grape berries can be infected through the stoma and lenticels of the pedicel and at a lower extent of the rachis. Pedicel infections are possible during the flowering period too. Later this tissue increases resistance against B. cinerea infections.
Other infection pathways postulating the saprophytic growth of the pathogen on floral debris and the later infection of berries when the susceptibility increases with ripening or by insect or hail damage of the berries. The assumption of conidia accumulation within the fruit during summer and the infection of susceptible berries later in the season is another thesis. Conidial infection of ripening fruit is possible from any inoculum source. Most probably a low number of latently infected berries are formed, which show extensive sporulation when the susceptibility of the berries increases with maturity. With can assume berries to become susceptible starting from a sugar content of 6%.
In kiwi fruit, we have a big impact of picking conditions to the occurrence of B. cinerea. Fruits picked with a wet surface can get infected by B. cinerea at the micro lesions set by the fingers of the pickers.
In practical control of B. cinerea we have to separate two important infection periods: Flowering and senescence. 1) During flowering we have susceptible young fruits, where the infection is followed by a latency period. 2) While infection on matures (senescent) fruits will lead to symptoms without a latency period. The importance of the infection during flowering in grapes can change from season to season and between region. In fruits where we have to expect some shelf live (table grapes, kiwis or strawberries) the symptoms are seen when stored under cooled conditions in shops or storages. Chemical control of vine grapes showing good resistance to B. cinerea during the flowering will not show any economic results. Therefore, all conditions of risk and probability of infection, the susceptibility of the fruit and the shelf life, storage conditions have to be taken into consideration in the decision of an application against Botrytis cinerea during flowering.
In stone fruits infection by B. cinerea occurs mostly during flowering. At this time treatments against Monilina spp. are taken into considerations which also infect the Botrytis cinerea infection.
Model of B. cinerea and practical use
Sensors needed: Leaf wetness, temperature, relative humidity
Botrytis cinerea is a facultative parasite. It grows on dead plant material too. Because of this fact it is always present in vineyards and orchards. Botrytis cinerea is related to a moist climate. For infection, it needs very high relative humidity or the presence of free water (sensor: leaf wetness, relative humidity). The fungus is unable to infect healthy adult plant material by spores. Infection takes place on young shoots of the vine during longer wet periods or shoots damaged by hail storms.
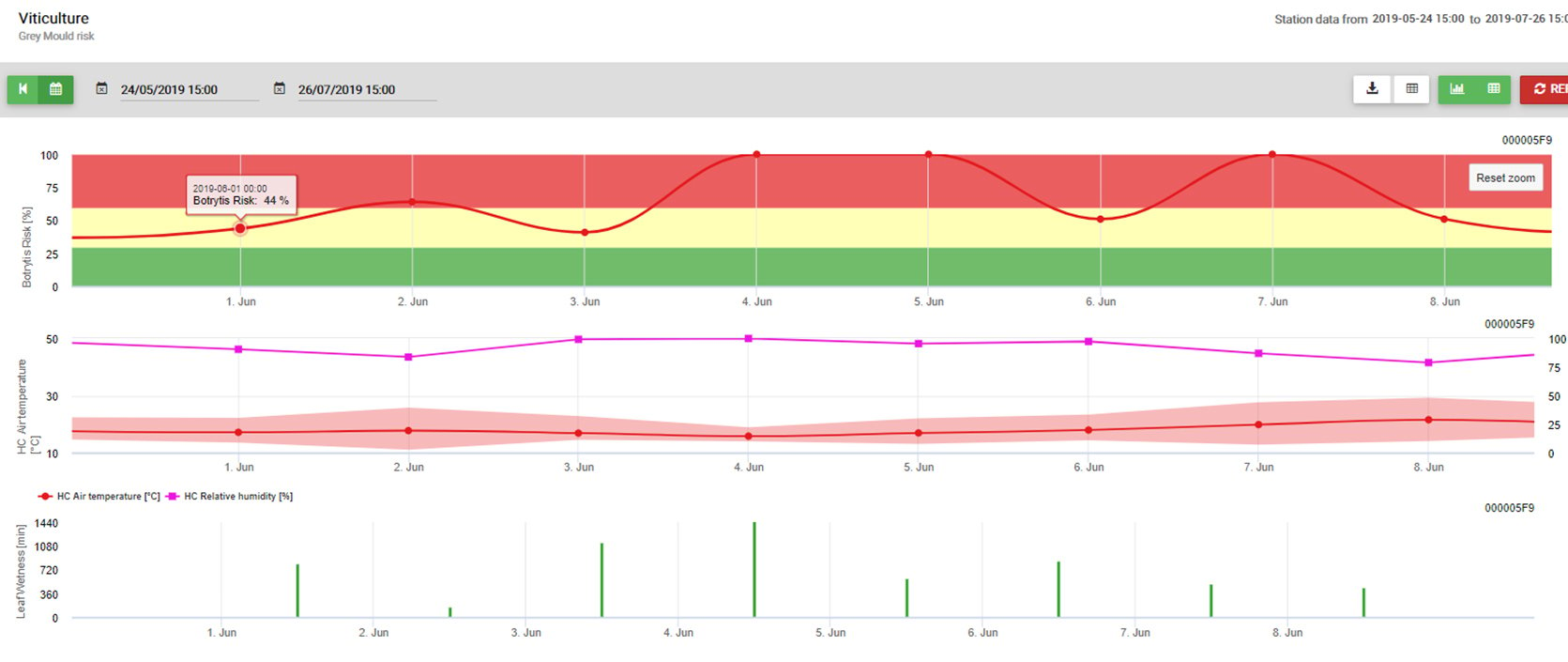
The model use the following correlation between leaf wetness duration and temperature to calculate the risk of an infection.
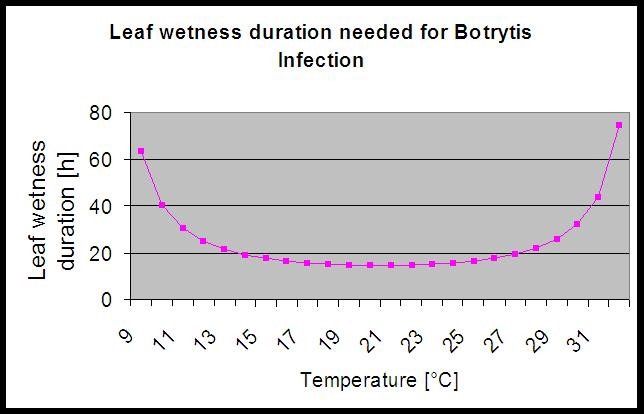
The Botrytis Risk Model results in a risk value of 0 to 100%. This value indicates the pressure of B. cinerea at the time. If we have a value of 100% it means that there has been several times a wetness period long enough to infect the susceptible tissue (we calculate so-called “wet points” (array between leaf wetness, temperature with a maximum of initially 38400 points (beginning of season, which displays 30% risk). After this period each wet period with about 4000 wet points (array) increase the risk by 10% or on the other side each dry period reduce the risk by 1/5 of the former value.
An application against B. cinerea is depending on the fruit and the production target.
Sclerotinia rot
Sclerotinia rot affects a wide range of plants particularly non-woody species. Sclerotinia rot is caused by S. sclerotiorum. Sclerotinia rot can affect plants at any stage of production including seedlings, mature plants and harvested products. Plants with senescing or dead tissue are particularly susceptible to infection.
Symptoms
The infected area of a plant initially takes on a dark green or brown water-soaked appearance, then may become paler in color. Dense white cottony mycelium usually develops and the plant begins to wilt and eventually dies. Resting or survival structures (sclerotia) are produced externally on affected plant parts and internally in stem pith cavities. The sclerotia are hard, black, irregular in shape, mostly 2-4 mm in size, and difficult to see once incorporated into the soil.
Disease sources and spread
The life-cycle of S. sclerotiorum includes both a soil-borne and an air-borne phase. Sclerotia of S. sclerotiorum can survive in the soil for ten years or more. They germinate to produce small funnel-shaped fruiting bodies (apothecia) that are approximately 1 cm in diameter. Apothecia produce air-borne spores, which can cause infection when they land on a susceptible host plant, either via flowers, or by direct germination on leaves. Occasionally, infection of stem bases can occur when fungal strands (mycelium) develop directly from Sclerotia near the surface. New sclerotia develop in infected plant tissue and when the plant dies they remain on the soil surface or may become incorporated during subsequent soil cultivation.
Conditions for Infection
After a period of cold conditions in winter, sclerotia, overwintering in the top 5 cm of the soil, germinate from spring onwards to produce apothecia, when soil temperatures are 10°C or higher and the soil is moist. Sclerotia do not germinate in dry soil or when the soil temperature is above 25°C. Sclerotia buried below 5 cm in the soil are less likely to germinate. Once apothecia are fully formed, spore release can occur in the light or dark but is temperature dependent, so tends to peak around midday. Apothecia can last about 20 days at 15 to 20°C but shrivel after less than 10 days at 25°C. For flowering herbs, spores landing on petals and stamens germinate rapidly (germination within 3-6 hours and infection within 24 hours) in optimum conditions of 15-25°C, continuous leaf wetness and high humidity within the crop. Subsequent infection of leaves and stems depends on petals falling and sticking on leaves. The risk of infection is increased if the leaves are wet because this causes more petals to stick. Infected dead or senescing petals provide nutrients for the invasion of the fungus into leaves and stems. For non-flowering herbs, infection is mainly by air-borne spores landing directly on leaves. Spores can survive on leaves for several weeks until conditions favorable for leaf infection occur. Spore germination and infection depend on the presence of nutrients on leaves, either from plant wounds or senescing plant material. As for flowering herbs, the optimum spore germination and infection conditions are 15-25°C with continuous leaf wetness and high humidity. Once plant infection has occurred, rapid disease progression is favoured by warm (15-20°C) and moist conditions in dense crops.
Sclerotinia Infection Model
Carpogenic germination of sclerotia is stimulated by periods of continuous soil moisture. Apothecia are formed on the soil surface from which ascospores are released into the air. Infection of most crop species is principally associated with ascospores but direct infection of healthy, intact plant tissue from germinating ascospores usually does not occur. Instead, infection of leaf and stem tissue of healthy plants results only when germinating ascospores colonize dead or senescing tissues, usually flower parts such as abscised petals, prior to the formation of infection structures and penetration. Myceliogenic germination of sclerotia at the soil surface can also result in the colonization of dead organic matter with subsequent infection of adjacent living plants. However, in some crops, for example sunflower myceliogenic germination of sclerotia can directly initiate the infection process of the roots and basal stem resulting in wilt. The stimulus for myceliogenic germination and infection in sunflower is not known but likely depends on nutritional signals in the rhizosphere derived from host plants.
The infection process
Infection of healthy tissue depends on the formation of an appressorium, which may be simple or complex in structure depending on the host surface. In most cases, penetration is directly through the cuticle and not through stomata. Appressoria develop from terminal dichotomous branching of hyphae growing on the host surface and consist of a pad of broad, multi-septate, short hyphae that are orientated perpendicular to the host surface to which they are attached by mucilage. Complex appressoria are often referred to as infection cushions. Although earlier workers considered penetration of the cuticle to be a purely mechanical process there is strong evidence from ultrastructural studies that enzymatic digestion of the cuticle also plays a role in the penetration process. Little is known about S. sclerotiorum cutinases, however, the genome encodes at least four cutinase-like enzymes (Hegedus unpublished). A large vesicle, formed at the appressorium tip prior to penetration, appears to be released into the host cuticle during penetration. After penetration of the cuticle, a subcuticular vesicle forms from which large hyphae fan outgrowing over and dissolving the subcuticular wall of the epidermis.
Infection by enzymatic degradation of the epidemic cells: Oxalic acid works in concern with cell wall degrading enzymes, such as polygalacturonase (PG), to bring about the destruction of host tissue by creating an environment conducive for PG attack on pectin in the middle lamella. This in turn releases low molecular weight derivatives that induce the expression of additional PG genes. Indeed, overall PG activity is induced by pectin or pectin-derived monosaccharides, such as galacturonic acid, and is repressed by the presence of glucose. Examination of the expression patterns of individual Sspg genes has revealed that the interplay among PGs and with the host during the various stages of infection is finely co-ordinated. (Dwayne D. Hegedus *, S. Roger Rimmer: Sclerotinia sclerotiorum: When ‘‘to be or not to be’’ a pathogen? FEMS Microbiology Letters 251 (2005) 177–184)
Looking for Climate Conditions for Infection of S. sclerotiorum has to take consideration of the apothecia formation, the sporulation, the direct infection by apothecia (even if it does not take place very frequently) and the infection from established mycelia by enzymatic degradation of the epidemic cells .
Apothecia formation and sporulation takes place if a rain of more than 8 mm is followed by a period of high relative humidity lasting longer than 20 hours at optimum temperature of 21°C to 26°C.
Direct Infection by Apothecia can be expected after a leaf wetness period followed by 16 hours of relative humidity higher than 90% under optimum 21°C to 26°C (“appressoria infection”). Whereas saprophytic growth followed by enzymatic degradation of the epidermic cells (“hydrolytic infection”) can be expected under a slightly lower relative humidity of 80% lasting for a period of 24 hours under optimum conditions of 21°C to 26°C.
Literature:
- Lumsden, R.D. (1976) Pectolytic enzymes of Sclerotinia sclerotiorum and their localization on infected bean. Can. J. Bot. 54,2630–2641.
- Tariq, V.N. and Jeffries, P. (1984) Appressorium formation by Sclerotinia sclerotiorum: scanning electron microscopy. Trans. Brit. Mycol. Soc. 82, 645–651.
- Boyle, C. (1921) Studies in the physiology of parasitism. VI. Infection by Sclerotinia libertiana. Ann. Bot. 35, 337–347.
- Abawi, G.S., Polach, F.J. and Molin, W.T. (1975) Infection of bean by ascospores of Whetzelinia sclerotiorum. Phytopathology 65, 673–678.
- Tariq, V.N. and Jeffries, P. (1986) Ultrastructure of penetration of Phaseolus spp. by Sclerotinia sclerotiorum. Can. J. Bot. 64, 2909– 2915.
- Marciano, P., Di Lenna, P. and Magro, P. (1983) Oxalic acid, cell wall degrading enzymes and pH in pathogenesis and their significance in the virulence of two Sclerotinia sclerotiorum isolates on sunflower. Physiol. Plant Pathol. 22, 339–345.
- Fraissinet-Tachet, L. and Fevre, M. (1996) Regulation by galacturonic acid of ppectinolytic enzyme production by Sclerotinia sclerotiorum. Curr. Microbiol. 33, 49–53.
Practical Use of the Sclerotinia Model
The White Leg Infection Model shows the periods when the formation of apothecia are expected. If these periods are consistent with the flowering period of rapeseed or canola we have to expect S. sclerotiorum infections during a moist period. The spores formed in the apothecia might be available for one to several days. The opportunity of infections is indicated by the calculation of the infection progress for direct or indirect infections by appressoria or enzymatic cell wall degradation. If the infection progress line reaches 100% an infection has to be assumed. These infections should be covered preventative or a fungicide with a curative action against S. sclerotiorum has to be used.
TomCast Alternaria
TOMCAST (TOMato disease foreCASTing) is a computer model based on field data that attempts to predict fungal disease development, namely Early Blight, Septoria Leaf Spot and Anthracnose on tomatoes. Field placed data loggers are recording hourly leaf wetness and temperature data. These data were analysed over a 24 hour period and may result in the formation of a Disease Severity Value (DSV); essentially an increment of disease development. As DSV accumulate, disease pressure continues to build on the crop. When the number of accumulated DSV exceed the spray interval, a fungicide application is recommended to relieve the disease pressure.
TOMCAST is derived from the original F.A.S.T. (Forecasting Alternaria solani on Tomatoes) model developed by Drs. Madden, Pennypacker, and MacNab? at Pennsylvania State University (PSU). The PSU F.A.S.T. model was further modified by Dr. Pitblado at the Ridgetown College in Ontario into what we now recognize as the TOMCAST model used by Ohio State University Extension. DSV are: A Disease Severity Value (DSV) is the unit of measure given to a specific increment of disease (early blight) development. In other words, a DSV is a numerical representation of how fast or slow disease (early blight) is accumulating in a tomato field. The DSV is determined by two factors; leaf wetness and temperature during the “leaf wet” hours. As the number of leaf wet hours and temperature increases, DSV accumulate at a faster rate. See the Disease Severity Value Chart below.
Conversely, when there are fewer leaf wet hours and the temperature is lower, DSV accumulate slowly if at all. When the total number of accumulated DSV exceeds a preset limit, called the spray interval or threshold, a fungicide spray is recommended to protect the foliage and fruit from disease development.
The spray interval (which determines when you should spray) can range between 15-20 DSV. The exact DSV a grower should use is usually supplied by the processor and depends on the fruit quality. Following a 15 DSV spray interval is a conservative use of the TOMCAST system, meaning you will spray more often than a grower who uses a 19 DSV spray interval with the TOMCAST system. The trade off is in the number of sprays applied during the season and the potential for the difference in fruit quality.
Studies have been initiated at Michigan State University to test the disease forecasting system, TomCast, for use in managing foliar blights on a carrot. TomCast has been used commercially in tomato production and has recently been adapted for use in disease management of asparagus. Processing carrots ‘Early Gold’ were planted with a precision vacuum seeder at the MSU Muck Soils Research Farm in three rows 18 inches apart on a raised bed that was 50 feet long. Carrot beds were spaced on 64-inch centres and in-row seed spacing was 1 inch. Each of the four replications of the experiment were located in separate blocks of carrots that consisted of 36 beds. Seventeen treatment beds 20 feet long were randomly placed in a checkerboard pattern in each replication. Treatments were applied with a CO2 backpack sprayer that was calibrated to deliver 50 gallons per acre of spray solution using 8002 flat fan nozzles. Treatments consisted of untreated and different schedule applications of Bravo Ultrex 82.5WDG (22.4 oz/A) alternated with Quadris 2.08SC (6.2 fl oz/A). The chemical program was applied on a 10-day calendar program as well as when predicted by the TomCast disease forecaster. Three different prediction thresholds of 15, 20, and 25 DSVs were used to time fungicide applications. When the cumulative daily DSV values reached the determined threshold a spray would be applied. Each treatment regime was initiated at four different levels of disease pressure (0%, trace, 5%, and 10% foliar blight). The first treatments were applied on 2 July and the last application of any treatment was made on 21 September. Ten feet of each centre row of the spray blocks were marked before the first application and were used for weekly disease ratings (see graphs, below). Yields were taken from the same ten feet section of row by hand-harvesting the carrots and topping and weighing.
This indicates that the first treatment in carrot should be done as soon as we can find the first disease incidence in the field. From now on it worked fine by the use of the TomCast model with a threshold of 20 DSV accumulated since the last spray.
Fieldclimate.com determines the severity of an Alternaria Infection in two different models:
Source: Jim Jasinski, TOMCAST Coordinator FOR OHIO, INDIANA, & MICHIGAN
Recommended equipment
Check which sensor set is needed for monitoring this crop’s potential diseases.