
Cherry disease models
Anthracnose
Pathogen
Anthracnose in cherries is caused by Colletotrichum sp.
The fungal pathogen overwinters in twigs and flower buds, with bud infections serving as a primary source of inoculum for emerging flowers and leaves. In spring, the fungus releases spores dispersed by rain and water splashes. Fruits can become infected at any stage of development, though symptoms become apparent only upon fruit maturation as the fungus begins to colonize the infected area and produce enzymes to degrade cell walls after remaining dormant. Cellular damage results in sunken, shriveled lesions on the fruit surface. Even spent fruit trusses can be infected post-harvest.
Symptoms
The disease initially manifests as small, water-soaked spots on leaves, which darken and enlarge over time. In early spring, conidia develop on the outer surface of bud and shoot scales.
Infected leaves often become distorted and curled and severe infections can lead to premature leaf drop.
Sweet cherry symptoms are limited to aborted and ripe, healthy fruits. Brown, mat-like lesions are produced on the fruit skin, growing to a diameter of 1-2 centimeters within a few days. Fruits can harbor asymptomatic infections during the green fruit stage.
FieldClimate model
General Colletotrichum Model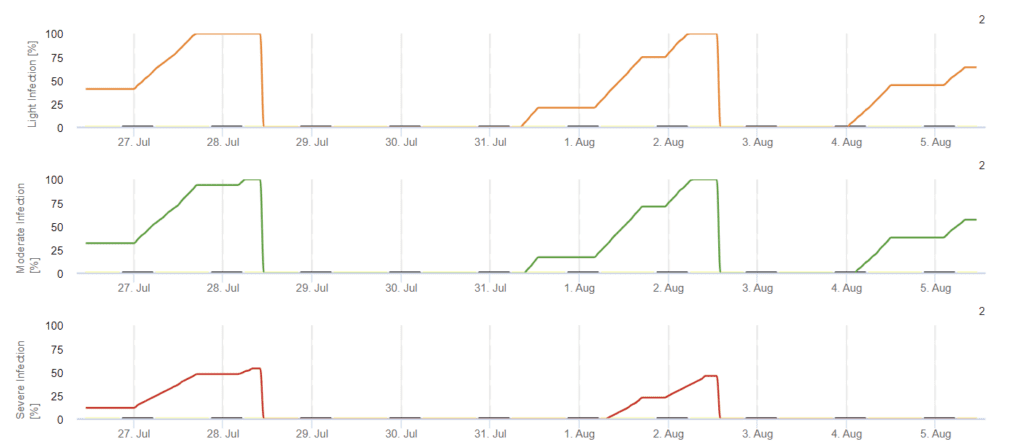
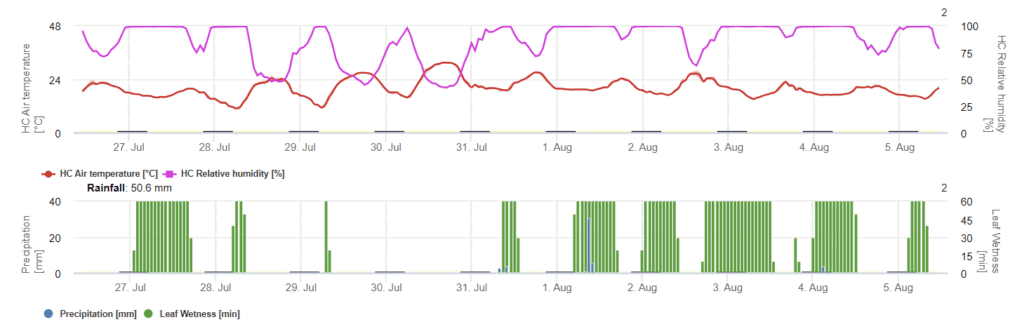
Sensors needed:
- Air temperature
- Relative humidity
- Leaf wetness
- Precipitation
In FieldClimate, weak, moderate, and severe infections are calculated in dependence on several parameters. Infections occur at a temperature optimum of 12- 27°C and a leaf wetness period of more than 12 hours. Whenever the graph reaches 100%, the conditions for a weak, moderate, or severe infection have been fulfilled. Considering plant varieties and location, the farmer should decide whether to take curative plant protection measurements.
Literature
- Børve, J., & Stensvand, A. (2006). Colletotrichum acutatum overwinters on sweet cherry buds. Plant disease, 90(11), 1452-1456.
- Børve, J., Djønne, R. T., & Stensvand, A. (2010). Colletotrichum acutatum occurs asymptomatically on sweet cherry leaves. European journal of plant pathology, 127, 325-332.
-> 이거는 한 번 더 읽기 - Tang, Z., Lou, J., He, L., Wang, Q., Chen, L., Zhong, X., … & Wang, Z. Q. (2022). First report of Colletotrichum fructicola causing anthracnose on cherry (Prunus avium) in China. Plant Disease, 106(1), 317.
- https://rjb.revistas.csic.es/index.php/rjb/article/view/61/60
-> 이 자료 pathogen으로 추가? - https://extension.umn.edu/plant-diseases/anthracnose-trees-and-shrubs
Powdery mildew
Pathogen
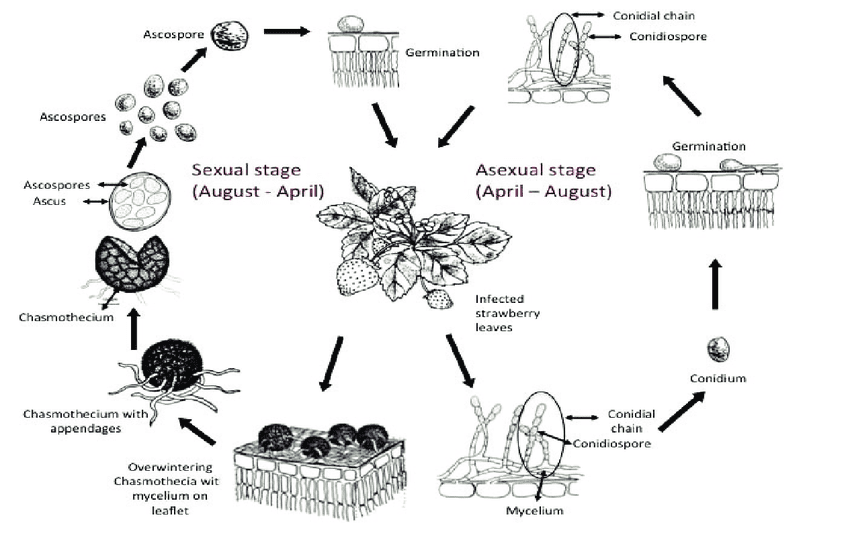
The pathogen of powdery mildew in cherries is Podosphaera clandestina. It overwinters as mycelia in buds and cleistothecia on leaves or tree crotches.
Primary infection occurs as ascospores (sexual spores) and conidia (asexual spores) are released and dispersed by wind and rain. They land on a new host, germinate, and form germ tubes and appressorium. The fungi feed themselves the fungi feed themselves via a haustorium by extracting the host’s nutrients.
Secondary infection occurs via conidia. After the initial infection, conidia are produced in large numbers on the infected tissue. These conidia are dispersed by wind and can rapidly spread the disease.
In the fall, as environmental conditions become less favorable, P. clandestina produces cleistothecia that can survive harsh winter conditions. When spring arrives, the spores are released and the cycle repeats.
Symptoms

The incubation period for powdery mildew typically lasts abut 7 to 10 days. Light white powdery patches develop on the surface of leaves, especially on younger, more susceptible leaves. This powdery mildew, composed of mycelium and spores, first appears on the underside of the leaves and spreads to both sides as the disease progresses. Infected leaves may become distorted and curl upwards.
Severe foliar infections can affect fruits too. Light infections appear around the stem bowl and heavily infected fruits may exhibit deformation and are covered with mycelium and spores. No infection of flowers has been observed.
FieldClimate Models
General Sphaerotheca pannosa Risk Model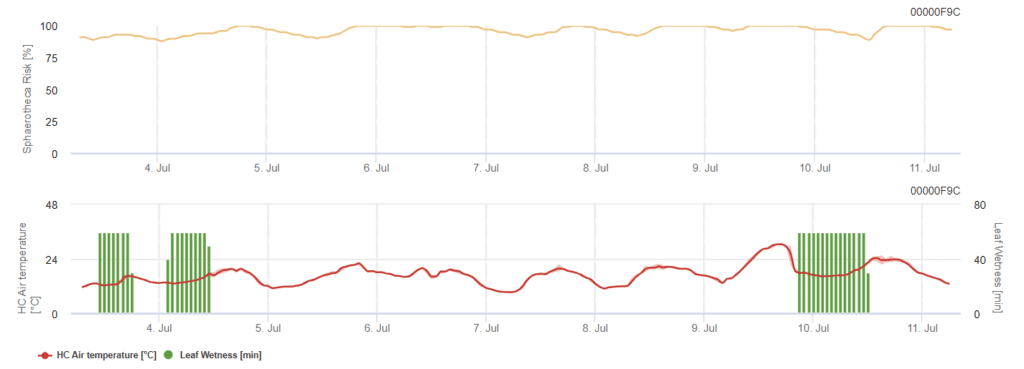
Sensors needed:
- Air temperature
- Leaf wetness
Powdery mildew fungi generally require moist conditions to release spores and infect plant tissue. No moisture, however, is needed for the fungus to germinate and grow after infection. It favors warm, Mediterranean climates.
Our model is based on leaf wetness durations and the temperature during this period. At a value over 60%, a high risk of an infection could be indicated and thus, plant protection measurements should be considered.
Literature
- Calabro, J. M. (2007). Biology of sweet cherry powdery mildew. Oregon State University.
- Jin XiaoLei, J. X., Fitt, B. D. L., Hall, A. M., & Huang YongJu, H. Y. (2013). The role of chasmothecia in the initiation of epidemics of powdery mildew (Podospheara aphanis) and the role of silicon in controlling the epidemics on strawberry.
- https://treefruit.wsu.edu/crop-protection/disease-management/cherry-powdery-mildew/
Recommended equipment
Check which sensor set is needed for monitoring this crop’s potential diseases.
