
Búza betegségmodellek
Búza robbanás
Kórokozó
Pyricularia grisea, más néven Magnaporthe oryzae, egy gombás kórokozó, amely a búza foltosságáért felelős, amely a búzanövényeket érintő jelentős betegség. A kórokozó elsősorban aszexuálisan, konídiumok (ivartalan spórák) útján szaporodik, amelyek a fertőzés elindításának elsődleges inokulumaként szolgálnak. Ezek a konídiumok konídiumfórákon termelődnek, és szél, eső és mechanikus eszközök, például mezőgazdasági gépek segítségével terjednek. A fogékony búza gazdaszervezeten landolva a konídiumok kicsíráznak és appressóriumokat képeznek - olyan speciális struktúrákat, amelyek megkönnyítik a növényi szövetekbe való behatolást. A gomba a belsejében kolonizálódik a gazdasejtekben, ami a betegség kialakulásához vezet. A környezeti feltételek döntő szerepet játszanak a fertőzés folyamatában. P. grisea. A gomba meleg, párás éghajlaton, 15 °C és 30 °C közötti hőmérsékleten és hosszabb ideig tartó levélnedves vagy magas relatív páratartalmú időszakokban fejlődik. A fertőzés megindulásához legalább 14 órán át tartó folyamatos levélnedvesség szükséges, így a túlzott felhőzet, a magas páratartalom, a túlöntözés és a rosszul időzített öntözés elősegíti a betegség kialakulását. Ezenkívül az olyan stressztényezők, mint a szárazság, a talajtömörödés, az alacsony kaszálási magasság és a túlzott nitrogéntrágyázás súlyosbíthatják a betegség súlyosságát.
Tünetek
A búzafoltosság kezdeti tünetei vízzel átitatott, rombusz alakú sérülések formájában jelentkeznek a leveleken, amelyek később a betegség előrehaladtával szürkévé válnak. Ezek a sérülések kiterjedhetnek és összenőhetnek, ami kiterjedt levélfoltossághoz és nekrózishoz vezet. A fertőzött hajtások részleges vagy teljes kifehéredést mutatnak, amely gyakran egy feketés-szürke fertőzési ponttól indul a hajtáscsúcson vagy a hajtás tövénél. Nagy inokulumnyomás esetén egyetlen tövön több fertőzési pont is kialakulhat, ami jelentős terméskiesést eredményez. A lombtünetek mellett, P. grisea a búzanövény más föld feletti részeit is megfertőzheti, beleértve a csomókat és a nyakat, ami rothadó nyak vagy csomórothadáshoz vezet. Ez a fertőzött részek letörését okozhatja, ami a termés elszáradását és további terméscsökkenést eredményezhet. A kórokozónak az a képessége, hogy a növény különböző részeit a vegetációs időszak során megfertőzheti, különösen kedvező környezeti feltételek mellett teszi különösen pusztítóvá.
FieldClimate modell
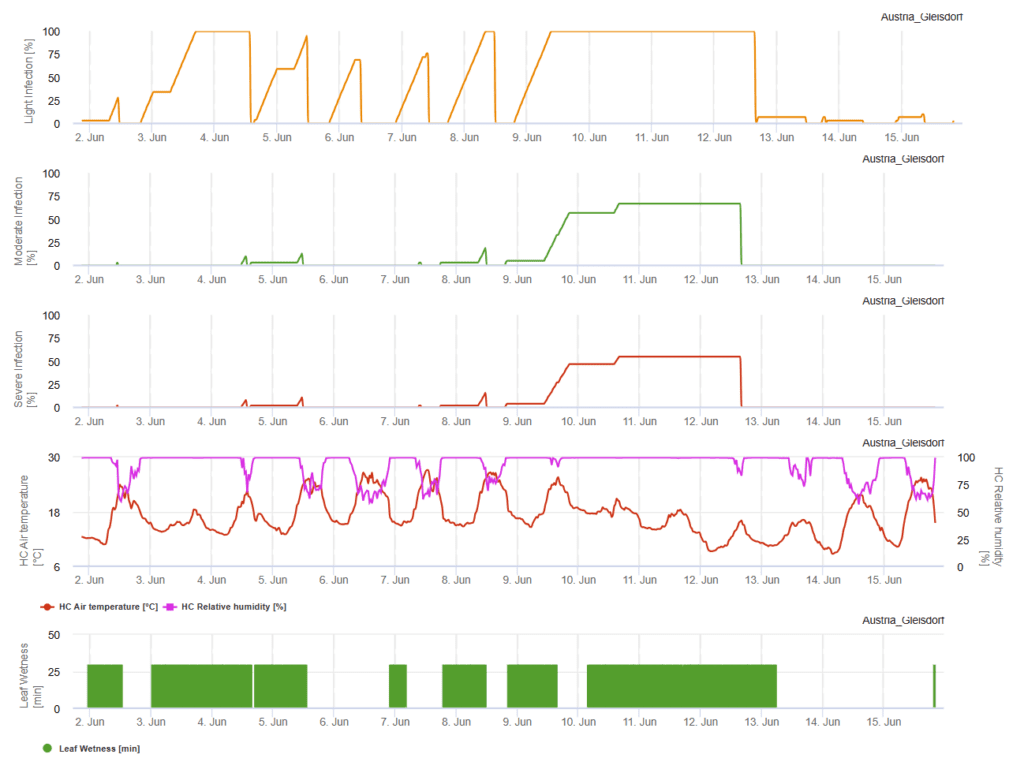
Szükséges érzékelők:
- Levegő hőmérséklete
- Relatív páratartalom
- Levél nedvesség
A felhős égbolt, a párás időjárás és a szitálás kedvez a betegség kialakulásának és súlyosságának. Az FieldClimate-ben három súlyossági osztályt számolunk, és amikor az 100% fertőzés elérte az optimális feltételeket a gombakórokozó számára a szántóföldön. A fajta függvényében, a szántóföldi előzmények alapján gyógyító növényvédelmi méréseket kell figyelembe venni, ha a könnyű, közepes vagy súlyos fertőzés elérte az 100% értéket.
Irodalom
- Cruz, C. D., & Valent, B. (2017). Búza foltos betegsége: mozgásban lévő veszély. Trópusi növénykórtan, 42(3), 210-222.
- Islam, M. T., Croll, D., Gladieux, P., Soanes, D. M., Persoons, A., Bhattacharjee, P., ... & Talbot, N. J. (2016). A búzafoltosság bangladesi megjelenését a Magnaporthe oryzae egy dél-amerikai vonala okozta. BMC Biology, 14(1), 84.
Pyrenophora teres
Kórokozó
Pyrenophora teres (Drechslera teres) két formában létezik - P. teres f. teres és P. teres f. maculata amelyek háló alakú hálófoltosságot, illetve folt alakú hálófoltosságot idéznek elő.
A pszeudotéciákból felszabaduló aszkospórák fertőzik a maradványokat, és elindítják az elsődleges fertőzést. A magról származó micéliumok és konídiumok egyes esetekben szintén elsődleges inokulumként szolgálnak. A spórák a leveleken kicsíráznak és áthatolnak a külső epidermális sejtfalon, így a kórokozó egy nagy intracelluláris vezikulában fejlődhet. Ezt követi az aszexuális szakasz, amelynek során konídiumok termelődnek, és a másodlagos fertőzést váltják ki, növelve a betegség súlyosságát. A tenyészidőszak végén a kórokozó pszeudotéciumokat termel, amelyek túlszaporodási forrásként a fertőzött árpatörmeléken maradnak.
Tünetek
A tünetek a leveleken, a szárakon és a magokon jelentkeznek, a betegség a növények aljától a teteje felé haladva. Az idősebb növények általában kevésbé súlyos károkat szenvednek, mivel vastagabb hámréteggel rendelkeznek, amely megakadályozza a kórokozó behatolását, és nagyobb mértékben képesek gombaellenes vegyületeket termelni. A kórokozók toxinokat termelnek, amelyek a tünetekért felelősek, hozzájárulnak a nekrózishoz és a klorózishoz, illetve sejt szinten megbontják a vízháztartást.
A hálós foltosság esetében a tünetek keskeny, sötétbarna, hosszanti irányú elváltozások formájában jelentkeznek. A nagyon ellenálló fajtákon csak néhány apró, pöttyszerű elváltozás alakul ki, és nem alakul ki kifejezett hálószerű mintázat. Ezzel szemben a legtöbb fogékony fajtán a nekrotikus elváltozásokat körülvevő klorotikus vagy vízzel átitatott területek jelenhetnek meg.
A foltos foltosság esetében a tünetek sötétbarna, kör alakú vagy elliptikus elváltozásokból állnak, amelyeket gyakran különböző szélességű klorotikus vagy nekrotikus halo vesz körül. A kevésbé fogékony fajtáknál a sérülések általában kisebbek, és hiányozhat a környező klorotikus halo.
A súlyos fertőzések a levelek teljes elhalását eredményezhetik, ami miatt azok száraznak tűnnek. Az idősebb levelek általában először hervadnak, majd a fiatalabbak következnek.
FieldClimate modell
Drechslera teres Modell
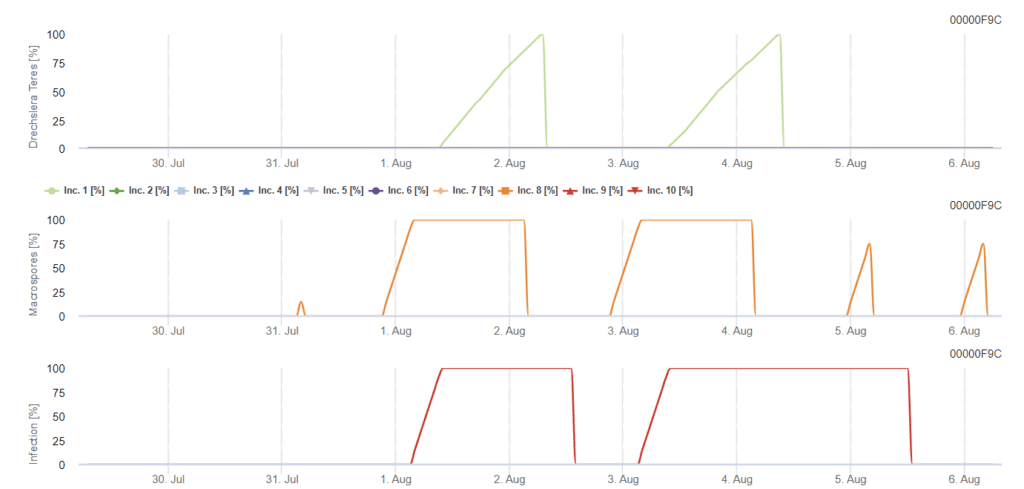
Szükséges érzékelők:
- Levegő hőmérséklete
- Relatív páratartalom
- Levél nedvesség
- Csapadék
Az elsődleges inokulum 15°C és 25°C közötti optimális hőmérsékleten fejlődik, az optimális hőmérséklet 20°C. A 6 óránál hosszabb sötétségi körülmények a konídiumok optimális növekedéséhez vezetnek, amint azt a konídiumok sporulációs grafikonja mutatja. Ha a sporulációs grafikon eléri az 100% értéket, akkor a területen optimális körülmények uralkodnak.
A további terjedés szél vagy eső útján történik. A növényi szövetek fertőzéséhez nedves körülményekre van szükség, mint például a levélnedvesség vagy a magas relatív páratartalom, körülbelül 10-30 órán keresztül, 15°C és 25°C optimális hőmérséklet mellett, ahogyan azt a fertőzési grafikon mutatja. Ha eléri az 100% értéket, akkor megállapítható, hogy a fertőzéshez a szántóföldön optimális feltételeket mértek. A napfény is fokozhatja a konidofórák növekedését, amelyek a magas napsugárzással és alacsony relatív páratartalommal járó nedves és száraz időszakok megváltozott körülményeit követően konídiumokat termelnek, és a szél is kedvez a betegség kialakulásának.
Irodalom
- Backes, A., Guerriero, G., Ait Barka, E., & Jacquard, C. (2021). Pyrenophora teres: rendszertan, morfológia, kölcsönhatás az árpával és a védekezés módja. Frontiers in plant science, 12, 614951.
- Liu, Z., Ellwood, S. R., Oliver, R. P., & Friesen, T. L. (2011). Pyrenophora teres: egy egyre nagyobb károkat okozó árpa kórokozó profilja. Molekuláris növénypatológia, 12(1), 1-19.
- Obst, A., & Paul, VH (1993). A gabonafélék betegségei és kártevői, Verlag Th. Mann, Gelsenkirchen-Buer.
Take-all
Kórokozó
A "Take-all" nevű betegség, amelyet a Gaeumannomyces graminis, a gabonafélék egyik legpusztítóbb betegsége. Az elnevezés a dél-ausztrál gazdákról származik, mivel a betegség annyira káros, hogy nem marad gabona, amit betakaríthatnának. A kórokozó károsítja a gyökérrendszert és a szárat, víz- és tápanyaghiányt okoz, ami végül a növény pusztulásához vezet.
A gomba micéliumként szaprofita módon él tovább a terménytörmelékben, és a talajból származó töredékek széllel, vízzel és állatokkal is elszállíthatók. Az elsődleges fertőzés akkor következik be, amikor a magoncok gyökerei fertőzött törmelékkel érintkeznek, majd ezt követi a hifák növekedése és terjedése. A gyökérhámon áthatolva és a gyökérkéregbe behatolva kolonizál és elpusztítja a szöveteket. A fertőzés felfelé és lefelé halad. A másodlagos fertőzés a gyökér-gyökér érintkezés útján történik, a súlyosan fertőzött növények foltokban fordulnak elő.
Tünetek
A búzában a Take-all betegség jellemzően a palántanevelési és a talajfelszín közelében okoz tüneteket. A fertőzött növények satnya, sárguló levelekkel és idő előtt érő, teljesen kifejlődött magvakkal rendelkező, korán érő kocsányokkal jelentkezhetnek. A néhány centimétertől akár méteres nagyságú, kör alakú elhalt foltok gyakran sárgás-narancsos vagy bronzos szélűek, és a következő években ugyanazon a területen újra megjelenhetnek.
A fő tünetek közé tartoznak a fehérre fakult vagy üres tüskék, a megfeketedett száralapok és a sötét, rothadó gyökerek, amelyeken apró, fekete, táguló sérülések láthatók. A súlyosan károsodott gyökerek törékenyek, és az alapszáron fényes fekete elszíneződés jelenhet meg. A növények a gyengült gyökérzetük miatt könnyen kihúzhatók a talajból. A betegség hasonlíthat a szárazság okozta stresszhez, mivel megszakítja a vízáramlást a növény felső részei felé, ami idő előtti fakadást okoz.
FieldClimate modell
Take-all
Modell érzékelők:
- Talajhőmérséklet
A take-all kockázati modell a naphossz és a talajhőmérséklet alapján határozza meg a kockázatos időszakokat. A 13 óránál rövidebb napsütéses napok és a 10 °C és 20 °C közötti talajhőmérséklet kedvez a betegség kialakulásának.
Ha a kockázati érték eléri az 100% értéket, a kórokozó fejlődéséhez optimális feltételek adottak a területen. A legfontosabb védekezési stratégia a vetésforgó, amelyben legalább 2-3 éves időközönként a fogékony gabonafélék között vetésforgó van.
Irodalom
- Cook, R. J. (2003). A búza összes felvétele. Fiziológiai és molekuláris növénykórtan, 62(2), 73-86.
- Palma-Guerrero, J., Chancellor, T., Spong, J., Canning, G., Hammond, J., McMillan, V. E., & Hammond-Kosack, K. E. (2021). Take-all betegség: új ismeretek egy fontos búza gyökérpatogénről. Trendek a növénytudományban, 26(8), 836-848. https://www.apsnet.org/edcenter/disandpath/fungalasco/pdlessons/Pages/Takeall.aspx. https://www.apsnet.org/edcenter/disandpath/fungalasco/pdlessons/Pages/Takeall.aspx
Fusarium head blight
Kórokozó
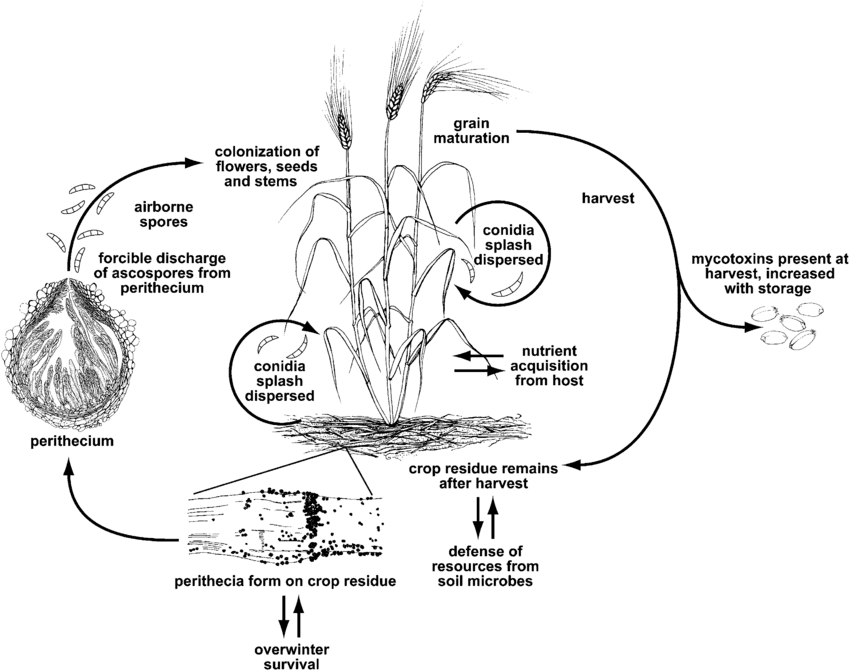
A fuzáriumos fejfoltosságot vagy a fuzáriumos foltosságot a következő nemzetség okozza Fusarium. A leggyakoribb faj a Fusarium graminearum de a legelterjedtebb fajok régiótól függően eltérőek lehetnek.
F. graminearum a búzanövényeket bármely növekedési szakaszban megfertőzheti. A telet a fertőzött növényi maradványokon vészeli át, tavasszal pedig az elsődleges fertőzés akkor következik be, amikor a peritéciákból és konídiumokból származó aszkospórák a búza bibére vagy porzóra szóródnak. A fertőzött virágzat zsugorodó vagy fonnyadt szemeket hoz létre, amelyeket gyakran "sírkő" szemeknek neveznek. Még ha a magok nem is tűnnek fertőzöttnek, akkor is lehetnek mikotoxinokkal szennyezettek. A másodlagos fertőzés konídiumokon keresztül történik, de a búza járványok elsősorban az elsődleges inokulum mennyiségétől, nem pedig a másodlagos forrásoktól függenek.
A fuzáriumos fejfoltosság káros mikotoxinok felhalmozódásához is vezet a terményekben, ami potenciális kockázatot jelent az állatokra nézve. Ezek a mikotoxinok hatástalanítják a növények védekező mechanizmusát, vagy védik a gombát más szervezetekkel szemben. A fő toxin a dezoxinivalenol (DON), amely gátolja a fehérjeszintézist, és megzavarja a sejtek normál működését. Azok az emberek, akik DON-t tartalmazó búzát fogyasztottak, olyan tüneteket tapasztalnak, mint hányinger, láz vagy hányás.
Tünetek

A búza és a durum esetében a fej bármelyik része vagy az egész fej kifehéredhet. A részben fehér és zöld fejek diagnosztikusak a betegségre búzában. A gomba a szárat is megfertőzheti, ami barnától a liláig terjedő elszíneződést okoz. Nedves körülmények között gyakran láthatók rózsaszínű vagy lazacnarancs színű spóratömegek a fertőzött tönkölyökön és búzaszemeken. Sok szem fonnyadt és könnyű, és néha "sírköveknek" nevezik őket meszes, élettelen megjelenésük miatt. Kedvező körülmények között a fertőzés átterjedhet a szomszédos tüskékre, és megfertőzheti az egész tüskét, beleértve a vesszőt és a szárat is. A magok tompa megjelenésűek és rózsaszínű elszíneződésűek lehetnek normál méretűek, ha a fertőzés a fejlődési szakasz végén következett be.
Az árpában a fertőzött tönk fehérré válik és vízzel átitatottnak tűnik. A magok is elszíneződhetnek, és nedves körülmények között esetenként lazacnarancs színű spóratömegek láthatók a tüskéken és a bibéken.
FieldClimate modellek
Az FHB szintjének becslése egy szántóföldön a foltos fejek vagy magok számán alapul. Az FHB súlyosságának becslésére szolgáló színes vizuális skála a búzában az NDSU Extension Service-től (PP-1095 kiadvány) érhető el: https://library.ndsu.edu/ir/bitstream/handle/10365/9187/PP1095_1998.pdf?sequence=1&isAllowed=y.
Fusarium head blight fertőzési modell (Fusarium head blight kockázat)
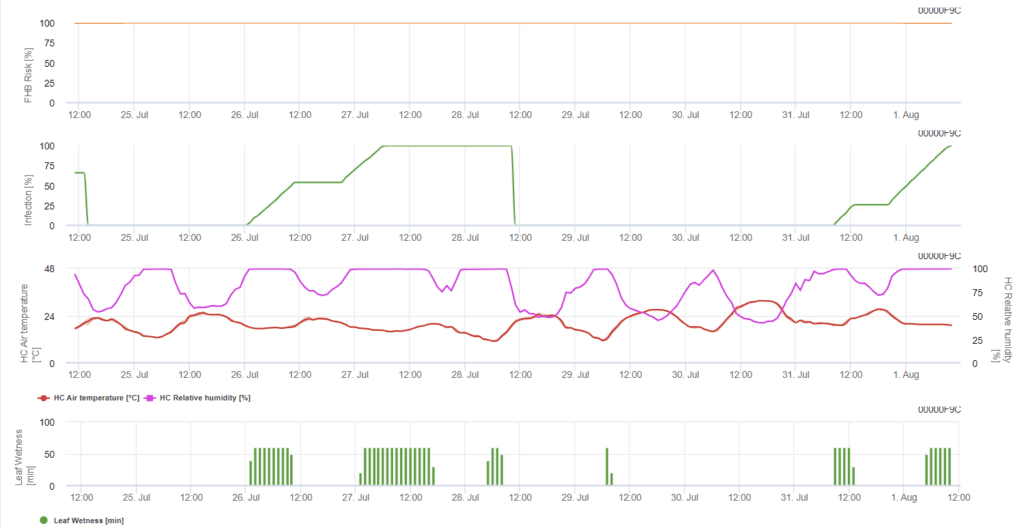
Szükséges érzékelők:
- Levegő hőmérséklete
- Relatív páratartalom
- Levélnedvesség
A modell meghatározza a fertőzés kockázati periódusait. Amikor az 100% fertőzést elérjük, a kockázat nagyon magas, és a gomba számára kedvezőek voltak a fertőzéshez szükséges feltételek. A gazdálkodó ismeretei a különböző búzafajták fejlődési stádiumáról lehetőséget adnak arra, hogy a fertőzés után azonnal döntsön a gyógyító permetezés alkalmazásáról.
A búzán előforduló Fusarium head blight gombakórokozóinak kedvez a 20°C és 30°C közötti meleg hőmérséklet és a hosszú párás időszakok. A többnapos levélnedves időszakok korai látható tünetekhez vezetnek. A tünetek hosszú látenciaidőszak után is jelentkezhetnek, ha a fertőzést 18 órás vagy még rövidebb levélnedvesítési időszak követi, valamint 15°C-os hőmérsékleten mesterséges beoltás után.
Összefoglalva a szakirodalomban talált különböző hőmérséklet- és nedvességkombinációkat, úgy döntöttünk, hogy a Fusarium fejfoltosság fertőzésekre akkor mutatunk rá, ha a hőmérséklet és a levélnedvesség periódusa vagy a 85%-nél nagyobb relatív nedvességtartalmú időszakok meghaladják a következő grafikonon látható értékeket. Az előrehaladási érték kiszámítása a nedves körülmények időtartama és a hőmérséklet közötti összefüggést követi.
Fusarium mikotoxin riasztási modell (Fusarium head blight)
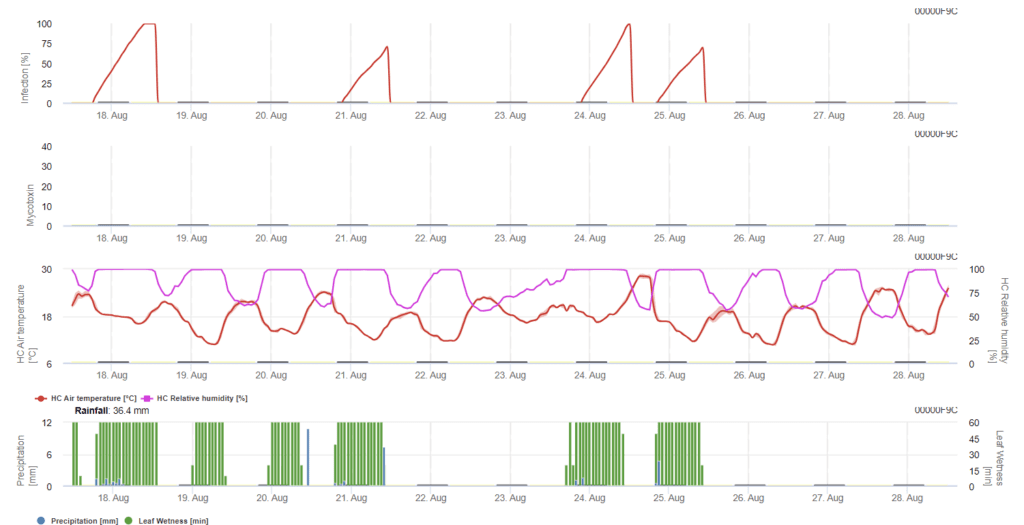
Szükséges érzékelők:
- Levegő hőmérséklete
- Relatív páratartalom
- Levélnedvesség
- Csapadék
ebben a modellben az FHB fertőzését a csapadék (2 mm szükséges), a relatív páratartalom (85% felett) vagy a levélnedvesség, a hőmérséklet alapján számítják ki a folyamat során. Ha a fertőzés eléri az 100% értéket, akkor a gombakórokozó számára optimális feltételek valósultak meg. A továbbiakban a modell kiszámítja az FHB mikotoxin kockázatát.
A Fusarium fejfoltosság hosszan tartó levélnedvességgel járó fertőzési kísérletek magas mikotoxin-tartalomhoz vezettek. Ezen információk alapján a 61. és 69. stádiumban 48 órás vagy annál hosszabb levélnedvesedési időszak feltételezhetően magas mikotoxin kockázatot jelent.
A kereskedelmi céllal termesztett búzában a DON vizsgálata során szerzett tapasztalatok azt mutatták, hogy a 61-69. stádiumban bekövetkezett kezdeti fertőzést követő, a fertőzéshez elég hosszú levélnedves időszakok növelhetik a DON-értékeket. Hosszabb levélnedvesedési időszakok esetén a mikotoxinok a 85. stádiumig emelkedhetnek.
- BBCH 61. szakasz: Virágzás kezdete; az első porzók láthatóak.
- BBCH 69. szakasz: A virágzás vége; az összes tüske virágzása befejeződött, de néhány kiszáradt porzó megmaradhat.
- BBCH 85. szakasz: Lágy tészta, szemtartalom puha, de száraz, körömlenyomat nem tartható.
A modell a számításhoz kiválasztott időszak alatt minden egyes sikeres fertőzéses időszakra vonatkozóan felhalmozza a fertőzés előrehaladásával arányos kockázati értéket. Hat befejezett fertőzés 100% kockázatot eredményezne. Általában a fuzáriumfertőzéshez vezető levélnedvesedési időszak hosszabb, mint a szükséges minimum. Ezért a legtöbb fuzáriumfertőzés 17%-nél nagyobb kockázatnövekedést eredményez.
A mikotoxin kockázati érték a szántóföldi előzmények alapján. A talajművelés nélküli búza után termesztett búza csak akkor hordozhat kis kockázatot, ha nem az optimális helyzetben permetezik. A permetezetlen búzában a 35% kockázat után megnövekedett DON értékekkel kell számolnunk. A nem talajműveléses búza után bármilyen más kultúrát követő búza nagyobb, 50% kockázatot hordozhat. Ha talajműveléses búzát követő búzánk van, a kockázat akár 70%-ig is megnövekedhet. Az elsőéves búzát DON-vizsgálatnak kell alávetni, ha a kockázat eléri az 100% értéket.
Irodalom
- https://www.fao.org/4/y4011e/y4011e0j.htm
- https://scabusa.org/pdfs/NDSU_PP-804_FHB-Small-Grains.pdf
- Lancashire, P. D., Bleiholder, H., Boom, T. V. D., Langelüddeke, P., Stauss, R., Weber, E., & Witzenberger, A. (1991). Egységes tizedes kód a növények és gyomok növekedési stádiumaira. Annals of Applied Biology, 119(3), 561-601.
- Trail, F. (2009). A gabonafélék foltos hullámaiért: Fusarium graminearum a posztgenomikai korszakban. Növényi fiziológia, 149(1), 103-110.
- Schumann, G. L. (2010). A növény-egészségügyi oktató| Volume: 10| Year: 2010| Article Type: Tantervek. Növényegészségügy, 10.
Árpa csík
Kórokozó
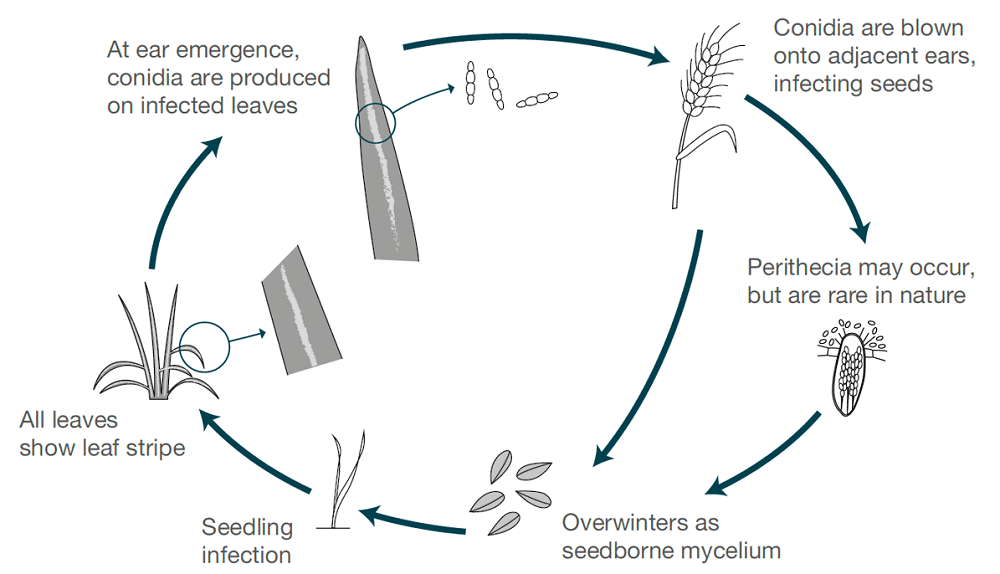
Az árpacsíkot a következők okozzák Pyrenophora graminea (Drechslera graminea), egy vetőmag által terjesztett kórokozó, amely a fertőzött vetőmag epidermiszében él tovább. A gomba hűvös, nedves körülmények között fertőzi meg a magoncokat. A gomba szisztémásan növekszik a növényen belül, megfertőzi az egész növényt, toxinokat termel, elpusztítja a sejteket, és elszínezi a levélszövetet az erek között, így csíkos elváltozásokat okoz. A csíkosodás az őszi árpán gyakoribb, mint a tavaszi árpán.
Ha a körülmények nedvesek vagy párásak, a spórák a levélfelületen termelődnek, amikor az egészséges növények tüskéi virágoznak. A spórák a szél által elszóródnak ezeken a fejlődő tüskéken, kicsíráznak és fertőzést okoznak. A magok a korai fejlődés során a legérzékenyebbek. Minden szezonban csak egy fertőzési és spóratermelési ciklusra kerül sor.
Tünetek

A tünetek főként a fül megjelenésekor jelentkeznek.
A halványzöldtől a sárgáig terjedő csíkok kezdetben a levél és a levélhüvely bazális részén jelennek meg. Ezek a csíkok fokozatosan barnává vagy sötétbarnává válnak, amit a levélnyél kiszáradása és felhasadása követ. A csíkok a levél teljes hosszára kiterjednek, nekrotikussá válnak és összeolvadnak, ami végül a növény pusztulásához vezet.
A növények satnyának tűnhetnek, kevés hajtást hoznak, súlyos esetekben nem hajtanak fejet vagy nem termelnek magot. A fül hossza is csökkenhet a rosszul fejlett barna szemek miatt.
FieldClimate modell
Pyrenophora graminea Modell
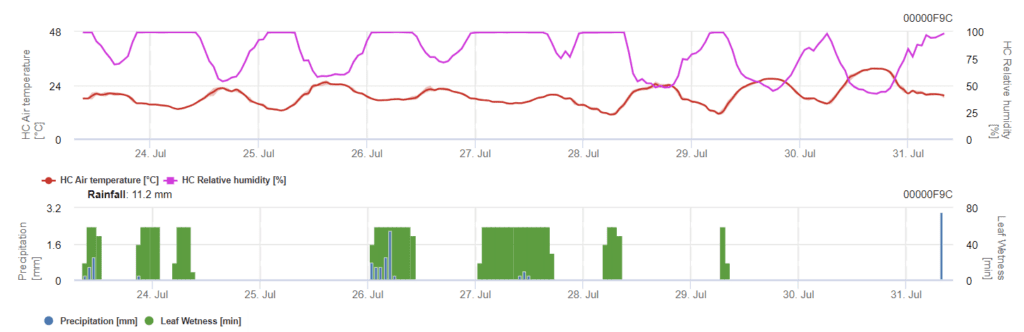
Szükséges érzékelők:
- Levegő hőmérséklete
- Relatív páratartalom
- Levélnedvesség
- Csapadék
A modellben két szakasz látható. Az első szakasz a kockázatos időszakok az üzem vészhelyzeti időszakai, korai szakaszai. A második szakasz a virágzási idő és az árpa fejfejlődésének ideje. Amikor a fertőzés eléri az 100%-t, a szántóföldön optimális feltételeket teremtettek ahhoz, hogy a gomba megfertőzze a növényi szöveteket és szisztematikusan növekedjen benne.
Irodalom
- Richardson, M. J. (1996). Magvak mikológiája. Mikológiai kutatás, 100(4), 385-392. https://ahdb.org.uk/knowledge-library/barley-leaf-stripe-life-cycle-and-disease-symptoms https://plantwiseplusknowledgebank.org/doi/full/10.1079/pwkb.species.46115.
Septoria
Kórokozó
A búzában két fő Septoria-betegség létezik: a Septoria tritici foltosság, amelyet a Septoria tritici okoz. Septoria tritici és a Septoria nodorum okozta foltosodás Septoria nodorum.
Septoria tritici a nyár folyamán a fertőzött növényi maradványokon él tovább, és ősszel kezdi megfertőzni a búzanövényeket. A gomba hűvös és nedves körülmények között fejlődik. Két szakasza van - egy látens és egy nekrotikus szakasz. A látens fázisban a gomba beoltja az új növényi szöveteket, és a sztómákon keresztül bejutva kolonizáció következik, amely során intercellulárisan növekszik a növényi szövetekben. S. tritici nem igényel fizikai táplálkozási struktúrákat, hanem úgy táplálkozik, hogy tápanyagokat von ki a gazdasejtekből, amelyek elvesztették szerkezeti integritásukat. A növények túlérzékeny reakciót mutathatnak, és a fertőzésre adott válaszként nekrotikus elváltozások alakulhatnak ki az érintett szöveteken.
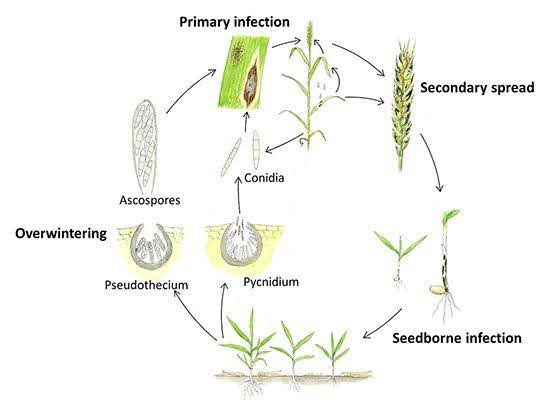
Septoria nodorum a nyár folyamán nyugvó micélium, piknídiumok és pszeudotéciumok formájában él tovább a növényi maradványokon. A fertőzés jellemzően ősszel kezdődik, amikor a piknídiumokból származó piknidiospórák és az álgombákból származó aszkospórák a szél és az eső által szétszóródnak, és elindítják az elsődleges fertőzést. Ezek a spórák behatolnak a levelek kutikulájába, és a fertőzést követően következik a piknídiumok képződése. A másodlagos fertőzés a piknídióspórák termelődésével és az alsó levelekről a felső levelekre és a levélkékre történő szétszóródásával következik be. A kórokozó toxinokat is termel, amelyek hozzájárulnak a betegség kialakulásához.
Tünetek
Septoria tritici foltosság
Ősszel, Septoria tritici a tünetek kezdetben apró sárga foltok formájában jelennek meg a leveleken, amelyeken apró fekete pöttyök találhatók, amelyek a gombák termőtestei. A sérülések szabálytalan alakúak, az elliptikustól a hosszú és keskenyig terjednek, megnagyobbodnak, és érésük során barnává vagy vörösesbarnává válnak. A betegség jellemzően az alsó leveleken kezdődik, majd fokozatosan felfelé halad, és végül a zászlóslevelet is érinti. Nedves körülmények között a gomba átterjedhet a búzafejre, és barna elváltozásokat okozhat a búzaszemeken és a levélnyélen, amit búzaszemfoltosságnak nevezünk. A Septoria tritici foltosság összetéveszthető más búzabetegségekkel. A fekete gomba testek jelenléte azonban a következők egyik fő jellemzője S. tritici. Bár a Septoria nodorum nedves körülmények között barna termőtesteket is képes létrehozni, ezek színükben és méretükben különböznek egymástól. Septoria tritici a termőtestek nagyobbak.
Septoria nodorum foltosság
Septoria nodorum a tünetek elsősorban a felső leveleken jelennek meg először. A leveleken kezdetben sötétbarna foltok jelennek meg sárga halóval. A levelek csúcsának égése egy másik kezdeti jel. A sérülések kiterjednek, és érésük során nekrotikussá válnak, sötétbarna középponttal. A sérüléseken belül apró sötétbarna struktúrák láthatók, amelyek termőtestek. A termőtestekből kiszabaduló konídiumok fehér vagy rózsaszínű tömegek. A súlyosan fertőzött leveleket teljesen beboríthatják a sérülések, ami végül a levélszövet elhalásához vezet. A gomba a levélkék és a levélnyelek is megbetegedhetnek: hasonló barna elváltozások jelennek meg, és a betegség lefelé halad.
FieldClimate modell
A Septoria-fertőzés alacsony hőmérsékleten is lehetséges, míg a 7 °C alatti hőmérséklet 2 napon belül nem feltétlenül vezet fertőzéshez. A betegség optimális hőmérséklete 16 és 21°C között alakul ki. A fertőzések a magas relatív páratartalom vagy a levélnedvesség 14 órás vagy annál hosszabb időszakában lehetségesek.
Septoria tritici modell
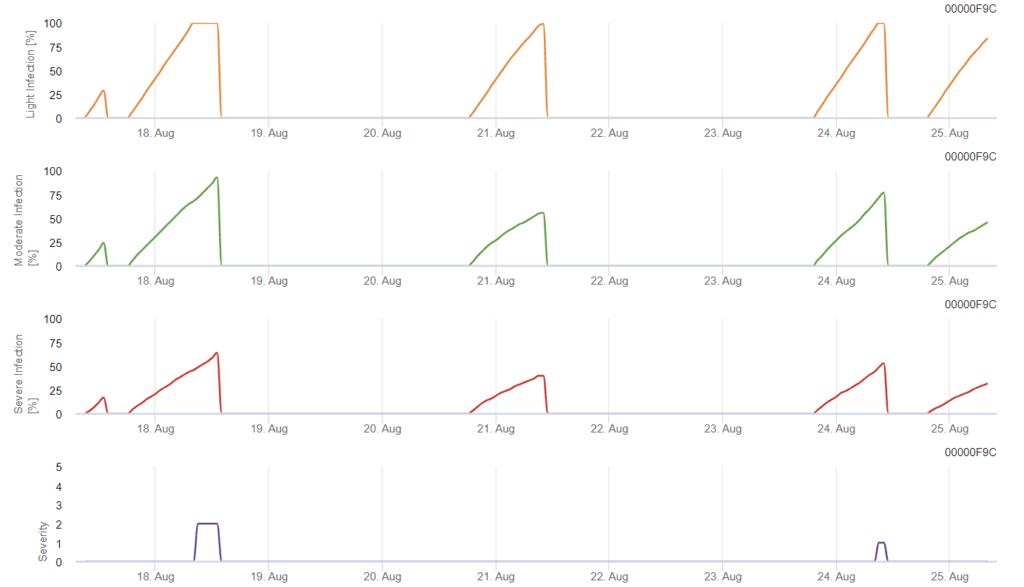
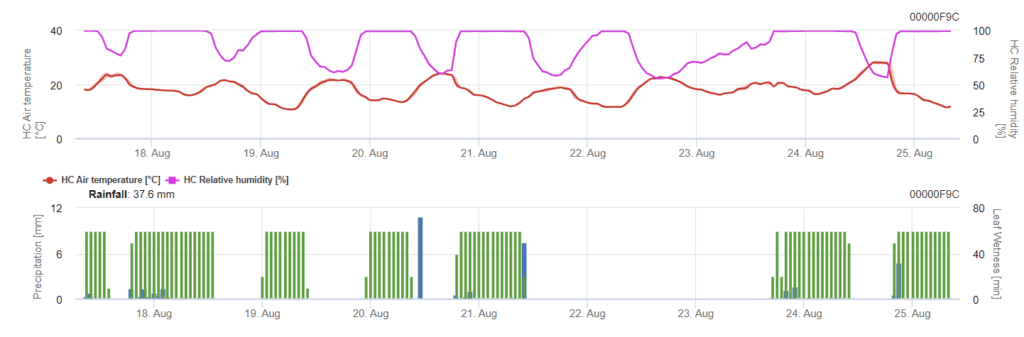
A fertőzés 0,5 mm-es eső után kezdődik. A Septoria-fertőzés optimális hőmérséklete 16°C és 21°C közötti hőmérséklet, valamint 14 órán át tartó levélnedvesség vagy magas relatív páratartalom. A Septoria tritici három súlyossági osztályba sorolható. Továbbá olyan súlyossági fokozatokat határozunk meg, amelyek a búza 10-32 BBCH-stádiuma közötti fertőzési nyomás értékelését segítik.
- BBCH 10. szakasz: Levélfejlődés; az első levél a koleoptiliumon keresztül
- BBCH 32. szakasz: A szár megnyúlása; a 2. csomópont legalább 2 cm-rel az 1. csomópont felett.
- BBCH 51. szakasz: A virágzat kibontakozása és a hajtás; a hajtás kezdete, amikor a virágzat csúcsa kibontakozik a hüvelyből, és az első tüske éppen csak látható.
Úgy döntöttünk, hogy nem használunk modellt a piknídiumképződésre. A piknídiumképződéshez szükséges feltételnek a 85%-nél magasabb relatív páratartalmú időszakot feltételezzük. A piknídiumok élettartama 24 óra. Minden olyan éghajlaton, ahol a gombának esélye van a fertőzésre, szinte minden nap napfelkelte körül találunk két órát, amely megfelel ennek a feltételnek.
A fertőzés súlyosságának értékelése
Az alábbiak felmérése Septoria tritici a fertőzési nyomás a 10. és 32. stádium között, valamint a 32. és 51. stádium között, a fertőzések súlyosságát az éghajlati viszonyok alapján kell megítélnünk. A fertőzés súlyosságát 1-5-ig terjedő skálán értékeljük:
- 1: gyenge fertőzés 5 mm-nél kisebb esővel
- 2: gyenge fertőzés több mint 5 mm-es esővel
- 3: mérsékelt fertőzés 5 mm-nél kisebb esővel
- 4: mérsékelt fertőzés 5 mm-nél nagyobb esővel/ súlyos fertőzés 5 mm-nél kisebb esővel
- 5: súlyos fertőzés 5 mm-nél nagyobb esővel
Betegségnyomás-értékelés
A szántóföldi betegségnyomást három tényező befolyásolja: az éghajlat, a szántóföldi előzmények és a termesztett fajta fogékonysága. Ha a betegség súlyossági értékeit a 10. stádiumtól a 32. stádiumig a 4-es értékre tudjuk felhalmozni, akkor az éghajlat által okozott gyenge betegségnyomásra számíthatunk. Ha ez az érték eléri a 6-os értéket, akkor mérsékelt betegségnyomásra számíthatunk, ha pedig eléri a 10-es értéket, akkor magasabb betegségnyomásra számíthatunk az éghajlat részéről.
A fajta fogékonyságának és a szántóföldi előzmények ismeretében a gyenge vagy mérsékelt betegségnyomás esetén a permetezés vagy annak mellőzése a helyzet függvénye. A 10-es kumulált érték mindenképpen a 32-es stádiumban történő permetezéshez vezethet.
A későbbi permetezésről szóló döntés a tavaszi időjárástól függ. Ha a 10. stádiumtól kezdve a súlyossági értékeket 6-os értékre tudjuk halmozni, akkor gyenge betegségnyomásra számíthatunk. Ha ez az érték eléri a 10-es értéket, akkor mérsékelt betegségnyomásra számíthatunk, ha pedig ez az érték eléri a 15-ös értéket, akkor az éghajlati helyzetből adódóan magas betegségnyomásra számíthatunk.
Az FieldClimate-ben a Septoria tritici súlyossága a három különböző fertőzési súlyossággal együtt jelenik meg. Az esőzések és a hosszú levélnedves időszakok miatt a S. tritici súlyos fertőzésének feltételei teljesültek. A súlyossági szintek elérik a legmagasabb, 5-ös értéket, ami azt jelenti, hogy a fertőzés veszélye magas.
Septoria nodorum modell
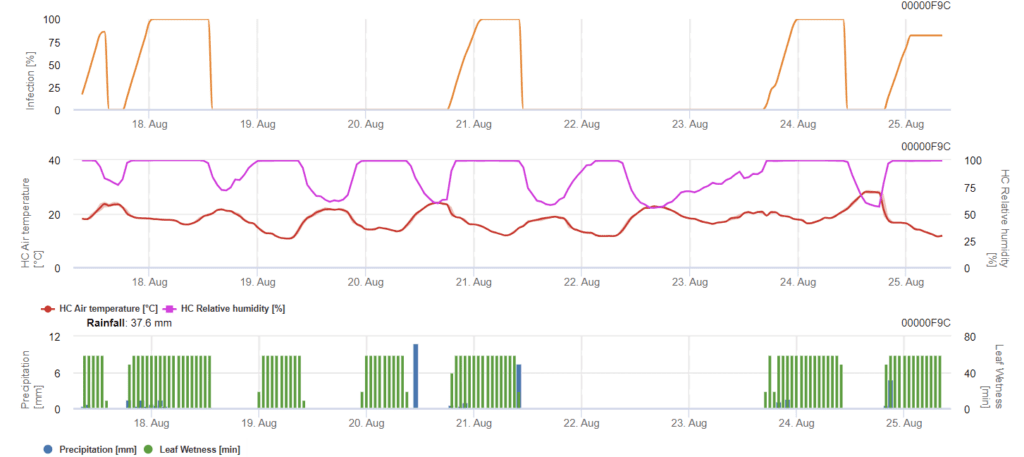
Septoria nodorumfertőzésbiológiája bizonyos mértékig különbözik a Septoria tritici de a különbség nem elég nagy ahhoz, hogy külön modellt alkossunk. Ezért javasoljuk a tritici modell használatát a Septoria betegség teljes komplexumára. Azokon a területeken, ahol nagy a nyomás a S. nodorum, a 2-es súlyossági értékkel gyengének minősített fertőzéseket komolyabban kell kezelni, mint más területeken.
A Septoria nodorum modell kiszámítja a betegség kockázatát. Növényvédelmi intézkedéseket kell mérlegelni, ha a kockázat eléri a 80% értéket. Ha a kockázat eléri az 100%-t és a fertőzés már megállapítható, szisztémás növényvédelmi méréseket (gyógyító alkalmazásokat) kell végezni.
Irodalom
- Brennan, C. J., Benbow, H. R., Mullins, E., & Doohan, F. M. (2019). A búza septoria tritici foltos betegségének korai szakaszában ismert ismeretlenek áttekintése. Növénykórtan, 68(8), 1427-1438.
- De Wolf, E. D. (2008). Septoria tritici foltosság. Mehra, L. K., Adhikari, U., Ojiambo, P. S., & Cowger, C. (2019). A búza Septoria nodorum foltossága. A növény-egészségügyi oktató.
- Solomon, P. S., Lowe, R. G., TAN, K. C., Waters, O. D., & Oliver, R. P. (2006). Stagonospora nodorum: a búza Stagonospora nodorum foltosságának oka. Molekuláris növénypatológia, 7(3), 147-156.
Levélfoltosság
Kórokozó
A levélfoltosságot a következők okozzák Rynchosporium secalis.
Az elsődleges fertőzés a növényi törmelékeken található aszkospórák vagy konídiumok útján történik. Ezeket a spórákat a szél és az eső szétszórja, kicsíráznak, és ahogy a fertőzést követően az epidermális és mezofill sejtek összeomlanak, a tünetek láthatóvá válnak. A másodlagos fertőzést a fertőzött levelekből származó konídiumok okozzák. Nedves körülmények között a konídiumok a levél felszínén csíráznak, és olyan hifákat hoznak létre, amelyek közvetlenül az epidermális sejtek fölött behatolnak a kutikulába. A későbbi gombásodás az epidermisz szubkutikuláris régiójára korlátozódik.
Tünetek
R. secalis a levelek bármely részét megfertőzheti. A szabálytalan alakú foltok a fő jellemzői, és a fertőzés az alsó levelektől a felső levelek felé halad. A foltok kezdetben vízzel átitatott területként jelennek meg. A betegség előrehaladtával a spóraképződés miatt középen szürkéssé válnak, barna szegéllyel. Nekrózis és klorózis következhet. A foltok összeolvadnak, és az egész levelet elpusztítják.
FieldClimate modell
Leaf Blotch Rynchosporium modell
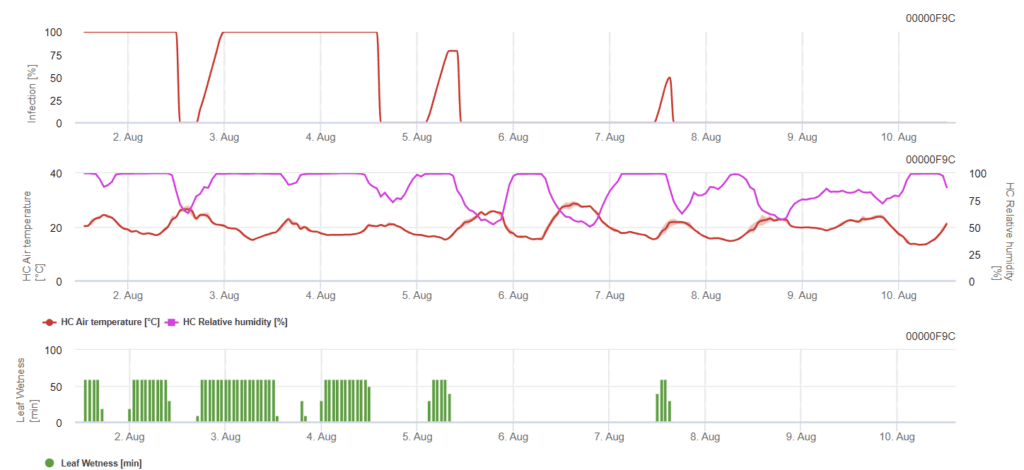
Szükséges érzékelők:
- Levegő hőmérséklete
- Relatív páratartalom
- Levélnedvesség
A fertőzés kialakulásához a hőmérséklettől függően legalább 7-15 óra ólomnedvességre van szükség (optimális 20'C-on). Amikor a grafikon eléri az 100% értéket, az azt jelenti, hogy a terepen optimális feltételeket határoztak meg.
Irodalom
- Brooks, F. T. (1928). Megfigyelések a Rhynchosporium secalis (Oud.) Davis, az árpa és a rozs levélfoltosságáról. New Phytologist, 27(4), 215-219.
- Fowler, A. M., & Owen, H. (1971). Az árpa levélfoltosságának (Rhynchosporium secalis) vizsgálata. Transactions of the British Mycological Society, 56.(1), 137-152.
- Zhan, J., Fitt, B. D., Pinnschmidt, H. O., Oxley, S. J. P., & Newton, A. C. (2008). A Rhynchosporium secalis populációk rezisztenciája, epidemiológiája és fenntartható kezelése árpán. Növénykórtan, 57(1), 1-14.
Ramularia levélfoltosság
Kórokozó
Ramularia collo-cygni, az árpa Ramularia levélfoltosságának kórokozója, elsősorban aszexuális szaporodás útján, konídiumok útján terjed, amelyeknek nedvességre van szükségük a csírázáshoz és a korai fejlődéshez. A levél felszínén történő csírázást követően a gomba a sztómákon keresztül jut be a levélbe, és intercellulárisan kolonizálódik a szövetekben. A fertőzött árpafélék kezdetben nem mutatnak tüneteket, de a károsodás jellemzően a virágzás után jelentkezik.
Az életciklus a R. collo-cygni nem teljesen tisztázott, de a fertőzött vetőmagokon keresztül történő vertikális átvitellel jár, ami lehetővé teszi a kórokozó túlélését a tenyészidőszakok között. A levegőben terjedő konídiumok szintén hozzájárulnak a másodlagos terjedéshez, kedvező körülmények között új fertőzéseket indítva el. Míg a magvak jelentik az elsődleges telelő mechanizmust, a helyettesítő gazdák és egy másodlagos gombaszerkezet, az ún. Asteromella továbbra is bizonytalan, és még tanulmányozás alatt áll.
Tünetek

A tünetek általában a levelek mindkét oldalán megjelennek a virágzás után, de a felső leveleken gyakoribbak. A kezdeti tünetek sárga vagy barna foltok formájában jelennek meg a levél erezeténél, amelyeket klorotikus halo vesz körül. A levélfoltok megjelenése után a levél klorotikussá és nekrotikussá válik, jellemzően a levél csúcsától és szélétől kezdve. A szomszédos foltok találkozhatnak, nagyobb sötét területeket hozva létre. Kis pontszerű foltok is megfigyelhetők. Bár a Ramularia levélfoltosság tünetei összetéveszthetők más betegségekkel, a legfontosabb jellemző, hogy a levélfoltok a levél erezetére korlátozódnak.
A Ramularia levélfoltok megkülönböztetésére úgynevezett 5R létezik:
- Sárga klorózisos peremmel gyűrűzött
- Téglalap alakú
- A levél erezete által korlátozott
- Vöröses-barna színezet
- Át a levélen
FieldClimate modell
Ramularia modell
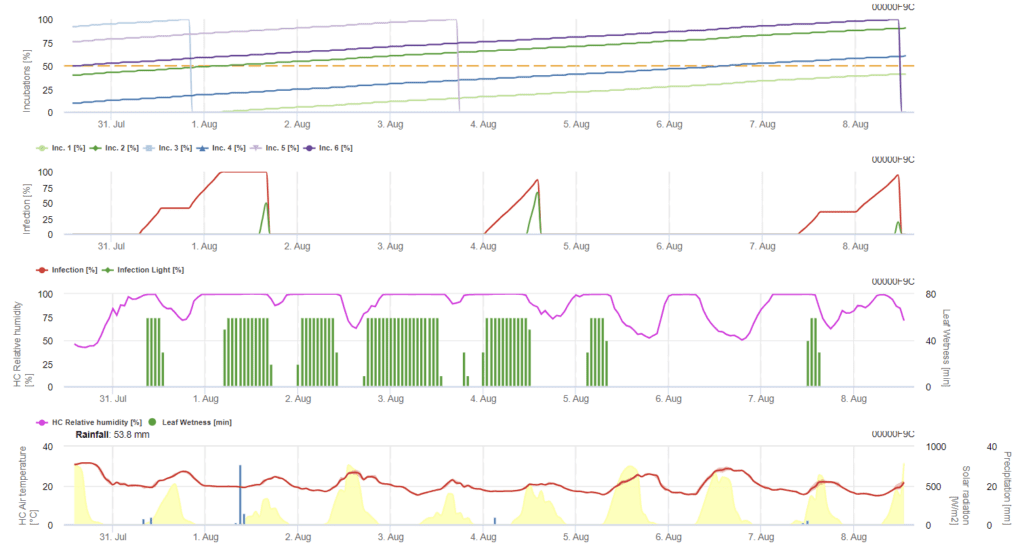
Az árpán a Ramularia-fertőzést leginkább a párás körülmények okozzák. Ezért a számításokat egy esőeseménnyel kezdjük, amelyet a 85%-nél magasabb levélnedvesség vagy relatív páratartalom követ.
A fertőzés kétféleképpen írható le:
- Fertőzés: egyszer, amikor a nedves körülmények csupán elősegítik a fertőzés kialakulását.
- Fertőzés: egyszer, amikor globális sugárzásra van szükség egy fertőzési esemény befejezéséhez.
A továbbiakban, amikor az 100% fertőzést elérjük, a modell elkezdi meghatározni az inkubációs időt. Amikor az inkubáció eléri az 50% értéket, a szántóföld ellenőrzése új inokulumok után javasolt, és amikor eléri az 100% értéket, a leveleken lévő nekrotikus elváltozások előrejelzése következik be.
Irodalom
- Havis, N. D., Brown, J. K., Clemente, G., Frei, P., Jedryczka, M., Kaczmarek, J., ... & Hess, M. (2015). Ramularia collo-cygni-an emerging pathogen of barley crops. Növénykórtan, 105(7), 895-904.
- Huss, H. (2002, április). A Ramularia collo-cygni biológiája. In Proc. Second Int. Workshop Barley Leaf Blights. Aleppo, Szíria (pp. 321-328).
- Walters, D. R., Havis, N. D., & Oxley, S. J. (2008). Ramularia collo-cygni: az árpa egy újonnan megjelenő kórokozójának biológiája. FEMS Microbiology Letters, 279.(1), 1-7. https://grdc.com.au/__data/assets/pdf_file/0025/443509/GRDC_FS2103_Ramularia_03.pdf
Rhizoctonia solani
Kórokozó
A Rhizoctonia gyökérrothadást a búzában a következő okozza Rhizoctonia solani. A kórokozó a talajban szkleróciumok formájában évekig is életben maradhat. Egyes esetekben micéliumként a növényi törmelékeken is fennmarad. Amint a hőmérséklet emelkedik, a szkleróciumok aktívvá válnak és hifák tömegét hozzák létre, ami a gyökerekhez tapadva lehetővé teszi a fertőzést. A micélium úgynevezett "fertőzési párnákat" hoz létre, és a gomba behatol a gyökérszövetbe, és ott kolonizál. A gyökérfertőzés gyengíti a csemetéket, ami gyenge növekedést eredményez, és csökkenti a víz és tápanyagok föld feletti növényi szövetekbe történő szállításának képességét, ami végül a növény pusztulásához vezet.
Tünetek
Korán megjelennek a csupasz foltok, amelyek mérete néhány centimétertől több méteres átmérőig terjed. Ezekhez a csupasz foltokhoz gyakran csonka növények társulnak, amelyek sárgulást, hervadást vagy akár lilás elszíneződést is mutathatnak. A fertőzött növények súlyosan elcsökevényesedhetnek, és a szárazság okozta stressz vagy tápanyaghiány jeleit mutathatják, ami egyes esetekben korai elhaláshoz vezethet.
Vörösesbarna elváltozások alakulnak ki a szárakon és a gyökereken a talajhatár alatt, és ezek az elváltozások a betegség előrehaladtával megsüllyednek. A gyökérrothadás csökkentheti a csomósodást, és a gyökérkéreg könnyen törhetővé válik, ami barna lándzsahegyeket eredményez.
FieldClimate modell
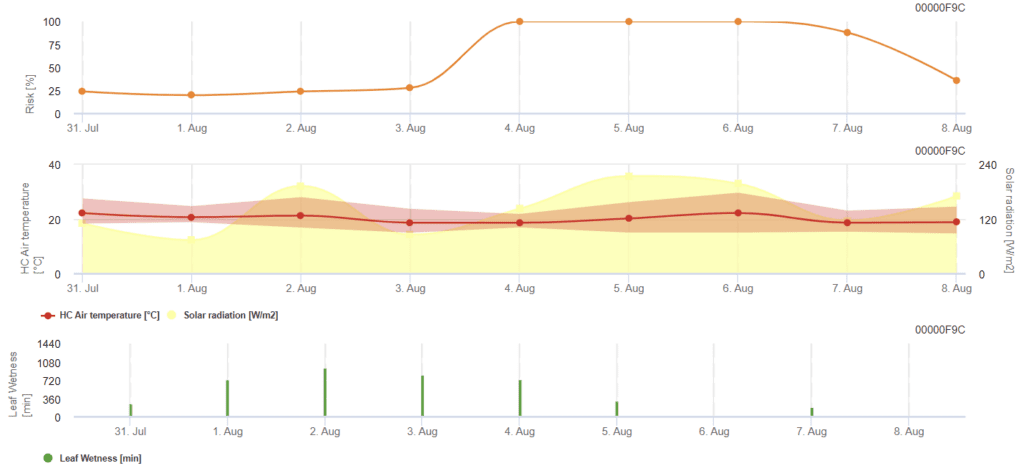
Rhizoctonia solani kockázati modell
Szükséges érzékelők:
- Levegő hőmérséklete
- Napsugárzás
- Levélnedvesség
A modell kiszámítja a kockázatos időszakok Rhizoctonia solani az elmúlt 120 óra körülményeinek ellenőrzésével. Az eredmény egy 0 és 100 közötti kockázati érték, amely a betegség szempontjából kedvező feltételeket jelzi.
Ha a kockázat alacsony, nincs szükség permetezésre. A mérsékelt kockázatú időszakokban a permetezési időköz meghosszabbítható, a magas kockázatú időszakokban pedig esetleg csökkenteni kell, vagy hatékonyabb vegyszerhasználat javasolt.
Egymást követő levélnedvesedés esetén percenként felhalmozza a hőmérséklettől függő értékeket:
- 12 °C és 15 °C között: percenként 1 felhalmozódás
- 16 °C és 17 °C között: percenként 2 felhalmozódás
- 18°C és több: 4 felhalmozódás percenként
A levélnedvesítési időszakok végén kiértékeli a felhalmozott értékeket:
- Értékek > 4096: Az értékből kivonva 4096-ot, a kockázat 64 ponttal nő.
- Maradékértékek > 2048: A kockázat 16 ponttal nő, és az értékből kivonják a 2048-at.
- Maradékértékek >1024: A kockázat 4-gyel nő, és az értékből kivonjuk az 1024-et.
Ha a globális sugárzás folyamatosan magasabb, mint 800 W/m², akkor a rendszer percekben felhalmozza az időt, és kiértékeli az értékeket, amikor a sugárzás alacsonyabb lesz:
- Érték > 512: Kockázat - 32 pont, érték - 512
- Érték > 256: érték - 256: Kockázat - 8 pont, érték - 256
- Érték > 128: Kockázat - 2 pont, érték - 128
Irodalom
- https://cropprotectionnetwork.org/encyclopedia/rhizoctonia-root-rot-of-wheat#:~:text=It%20is%20caused%20by%20Rhizoctonia,result%20in%20premature%20plant%20death.
- https://ahdb.org.uk/knowledge-library/rhizoctonia-stunt-symptoms-and-risk-in-cereals
- https://cropwatch.unl.edu/rhizoctonia-root-rot#:~:text=Disease%20Symptoms&text=These%20reddish%20brown%20lesions%20may,uneven%20because%20of%20stunted%20plants.
- https://extensionaus.com.au/FieldCropDiseasesVic/docs/identification-management-of-field-crop-diseases-in-victoria/soil-borne-diseases/rhizoctonia-root-rot/
- https://www.florimond-desprez.com/es/wp-content/uploads/sites/6/2015/11/rhizoctonia_eng.pdf
Lisztharmat
Kórokozó
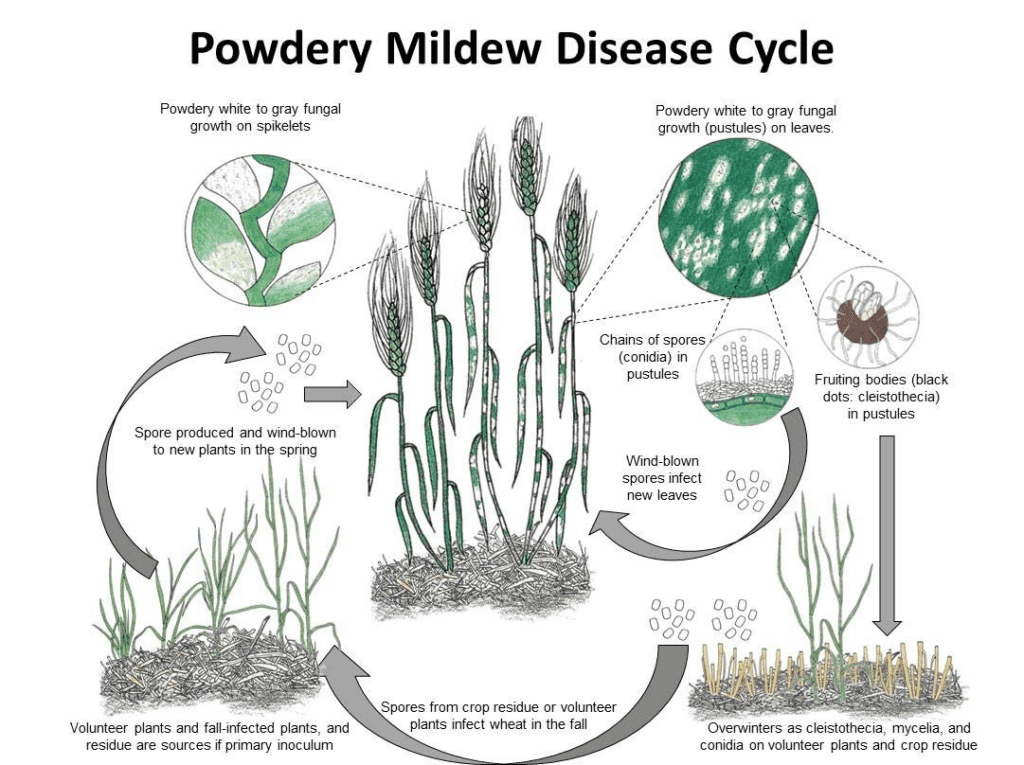
Blumeria graminis a búza és az árpa lisztharmatának kórokozója. Mizellumok vagy kleisztotéciumok formájában telel át. Tavasszal a spórák kicsíráznak és megfertőzik a gazdanövényeket hűvös és nedves körülmények között, anélkül, hogy a növényfelületeken szabad vízre lenne szükségük.
A gomba aszexuálisan és ivarosan is szaporodik.
Az aszexuális ciklusban a kórokozó gyors fertőzési és konídium (aszexuális spórák) termelődési ciklusokon megy keresztül, amelyek kulcsfontosságúak a betegség terjedésében. Kedvező körülmények között a micéliumban 7-10 naponként konídiumok termelődnek. A szél által szétszóródva ezek a konídiumok új gazdaszervezeten landolnak, kicsíráznak, és appressóriumokat és haustóriumokat képeznek, hogy tápanyagokat vonjanak ki a növényből. A Blumeria graminis konídiumai alacsony páratartalom mellett és különböző hőmérsékleten is képesek csírázni.
Az ivaros ciklusban a gomba kleisztotéciumokat termel, olyan szívós szerkezeteket, amelyek jól túlélik a kedvezőtlen körülményeket. A kleisztotéciumok aszkospórákat (ivaros spórákat) tartalmaznak, és e spórák felszabadulásakor új fertőzések indulnak el.
Tünetek

A lisztharmat a búzában és az árpában a szemtermést befolyásolja azáltal, hogy csökkenti a fejek számát, valamint a magok méretét és tömegét. Minél korábban történik a fertőzés, annál nagyobb a kár.
Az árpa korfüggő rezisztenciát mutat a lisztharmattal szemben. Ahogy az árpa érik, a jellegzetes micéliumfoltok helyett élesen körülhatárolt, fekete-barna foltok ("kátrányfoltok") jelenhetnek meg a leveleken.
A búza különösen érzékeny a lisztharmatra a kalászosodás és a tejérés időszaka között, különösen, ha a zászlóslevelek és a héjak érintettek. Az árpához képest azonban a búza jobban tűri a korai fertőzéseket.
A leveleken, szárakon és fejeken fehéres-szürkés lisztharmat alakul ki. A gombásodás leginkább a levelek felső felületén szembetűnő, bár az alsó oldalon is kialakulhat. A puszta kezdetben fehérnek tűnik, de érésük során fokozatosan szürkésbarnára színeződik, és nagyobb tömegeket alkotva, gyakran klorózissal körülvéve egyesülhet. A fertőzött levelek ellentétes oldalán a szövetek sárgák, később barnásbarnák vagy barnák lesznek. Cleistothecia is láthatóak, mint apró, kerek, fekete pontok a régebbi szürke telepeken belül. Súlyos esetekben a levelek elhalhatnak.
FieldClimate modell
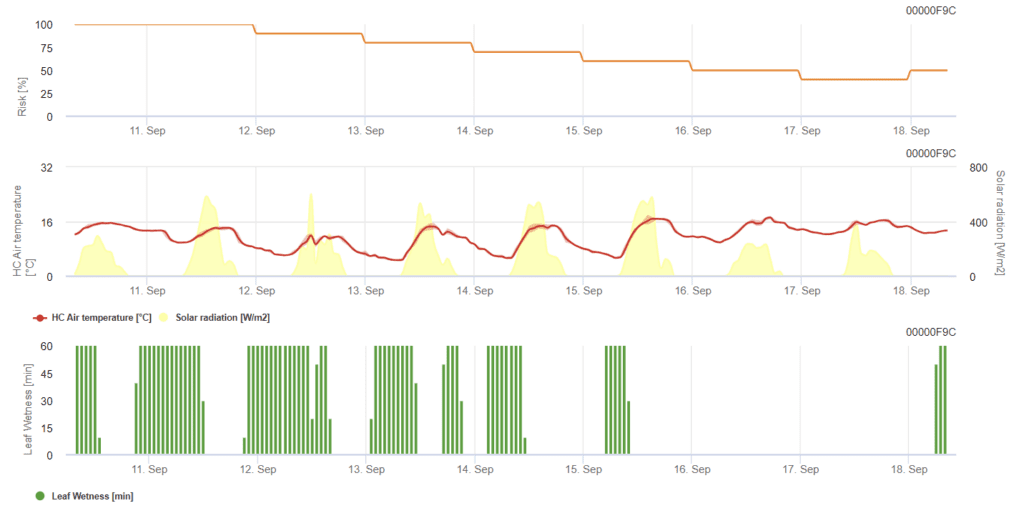
Búza lisztharmat modell
Szükséges érzékelők:
- Levegő hőmérséklete
- Levélnedvesség
- Napsugárzás
A modell a lisztharmat kockázatát 0-tól (nincs fertőzés) 100%-ig (teljes fertőzés) terjedő skálán határozza meg. A modell az árpa esetében a 21-39-es BBCH-stádiumban, a búza és a zab esetében pedig a 21-75-ös BBCH-stádiumban releváns.
A BBCH-stádium a növények fejlődésének leírására szolgáló növekedési skála. Két számjegyű, a skála 00-tól 99-ig terjed - a 00 a vetőmagkezelésre utal, a 99 pedig a betakarítás utáni kezelésre. Az első számjegy a fejlődés adott szakaszára utal; 0 a csírázás, 1 a levélfejlődés, 2 a csávázás, 3 a szárnynyúlás, 4 a bimbófejlődés, 5 a virágzat kialakulása és a fejtés, 6 a virágzás és az antézis, 7 a gyümölcsfejlődés, 8 az érés, 9 pedig az öregedés.
- BBCH 21: Tillering - A tillering kezdete; az első tiller kimutatható.
- BBCH 39: A szár megnyúlása - zászlóslevél stádium; a zászlóslevél teljesen kitekeredett, és a levélnyél éppen csak látható.
- BBCH 75: Gyümölcsfejlődés - közepesen tejes; szemtartalom tejes, a szemek elérik a végleges méretet, de még zöldek.
A kockázat a nap legtöbb órájában 12 °C és 21 °C közötti hőmérsékleten és alacsony globálsugárzás mellett nő. A levélnedvesség, a magas globálsugárzás és a 32 °C feletti magas hőmérséklet ezzel szemben csökkenti a kockázatot.
Irodalom
- Both, M., & Spanu, P. D. (2004). Blumeria graminis f. sp. hordei, az árpa obligát kórokozója. Éves Növényi Vélemények, 11, 202-218.
- Cunfer, B. M. (2002). Lisztharmat. Kenyérbúza: Javítás és termelés, 30, 317-330.
- Der Gräser, E. M. Krankheiten und Schädlinge des Getreides. Lancashire, P. D., Bleiholder, H., Boom, T. V. D., Langelüddeke, P., Stauss, R., Weber, E., & Witzenberger, A. (1991). Egységes tizedes kód a növények és gyomok növekedési stádiumaira. Annals of Applied Biology, 119(3), 561-601. https://ohioline.osu.edu/factsheet/plpath-cer-11. https://ohioline.osu.edu/factsheet/plpath-cer-11
Barna rozsda
Kórokozó
Három jelentős rozsda és kórokozó van:
- Levél/barna rozsda: Puccinia triticina
- Szár/ Fekete rozsda: Puccinia graminis
- Csíkos/ Sárga rozsda: Puccinia striiformis
A barna rozsda a búza leggyakoribb rozsdabetegsége. A fekete rozsdát nyári rozsdának is nevezik a bőséges fényes fekete teliospórák fejlődése miatt. Ez a legpusztítóbb rozsdabetegség, kedvező körülmények között egy hónap alatt a veszteségek 50 százalékát okozza. A sárga rozsda a búza betegsége hűvösebb éghajlaton, általában a magasabb fekvésű és északi szélességi körökhöz kötődik. Jellemzői a sárga színű urediniospórák. Súlyos esetekben a fekete rozsdához hasonlóan jelentős károkat okozhat.
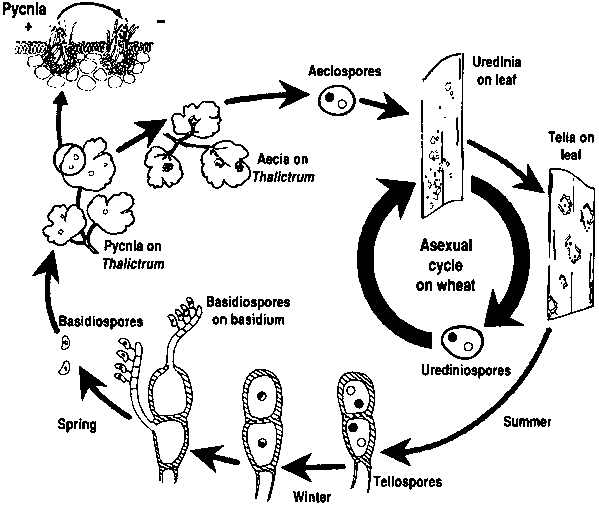
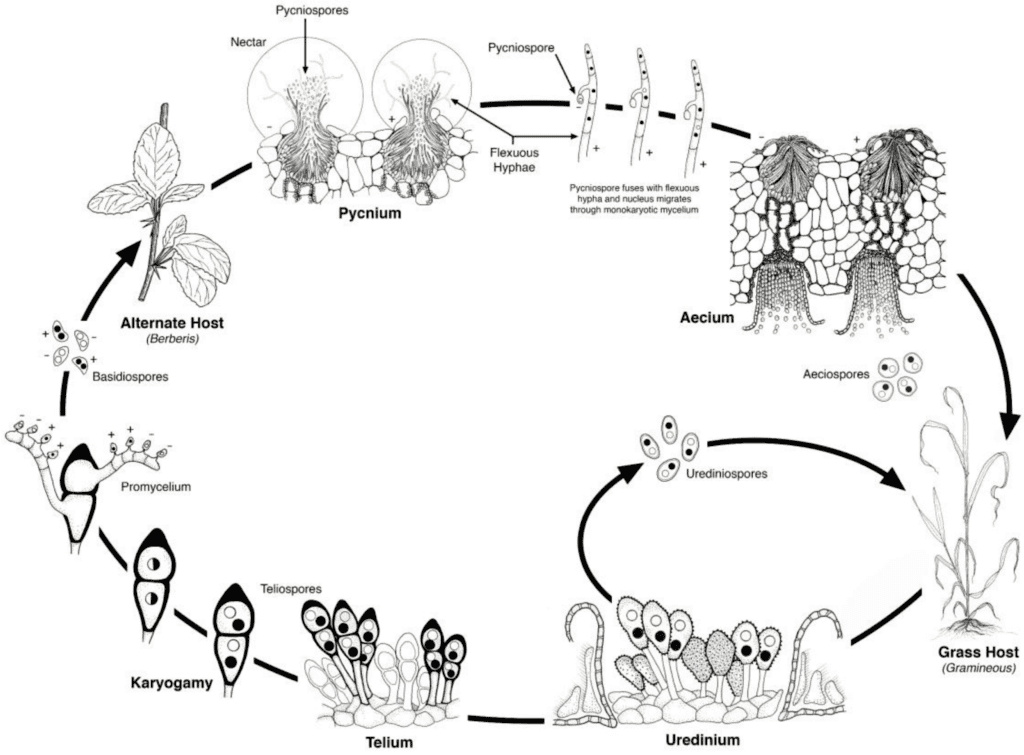
A búzarozsda kórokozói mind aszexuális, mind ivaros szaporodási cikluson mennek keresztül, és két gazdanövényt igényelnek - egy gazdasági gazdanövényt és egy helyettesítő gazdanövényt. A gazdasági gazda a búza, az alternatív gazda pedig jellemzően egy gyomnövény vagy őshonos növény, amely lehetővé teszi a gombák számára az ivaros fázist. Puccinia striiformis nem ismert, hogy lenne alternatív gazdája, uredinialis és telialis szakaszokból áll.
Az aszexuális ciklusban az urediniospórák a gazdanövényen termelődnek, és többször is megfertőzhetik azt, ami kedvező körülmények között gyors és széles körű fertőzéshez vezet.
A kórokozó ivaros ciklusa akkor kezdődik, amikor a gazdanövényen uredinális fertőzések során keletkező teliospórák kicsíráznak, és meiózison mennek keresztül, hogy bazidiospórákat hozzanak létre. Ezek a bazidiospórák szétszóródnak egy másik gazdaszervezetbe, ahol fertőzést indítanak el, amelynek eredményeképpen pikníoszpórákat és fogékony hifákat tartalmazó pikníoszpórák képződnek. A megtermékenyítés akkor következik be, amikor az egyik párosodási típusból származó picniospórák a nektár útján átkerülnek az ellenkező típusúba. A megtermékenyítést követően az alternatív gazdaszervezet leveleinek alján aeciospórák fejlődnek ki, amelyek aeciospórákat bocsátanak ki. Ezek az aeciospórák szétszóródnak és új gazdaszervezeteket fertőznek meg, majd uredinialis fertőzés következik, amely befejezi a kórokozó életciklusát.
Tünetek

A) Puccinia graminis
B) Puccinia striiformis
C) Puccinia triticina
Puccinia triticina
P. triticina elsősorban a levélkéket fertőzi, de esetenként a levélhüvelyeket, a levélkéket és a levélnyakakat is megfertőzheti, apró sárga foltokat képezve a levél felső felületén. Ezek a foltok a betegség előrehaladtával narancssárga színű pattanásokká fejlődnek, amelyeket sárga glória vesz körül. A pattanásokból a leveleken látható narancssárga vagy fekete spórák fejlődnek. A fekete rozsdától eltérően kevesebb teliospórát termel. Egyeseknél előfordulhatnak klorotikus vagy nekrotikus területekkel körülvett hiperérzékeny foltok vagy urediniumok.
Puccinia graminis
P. graminis főként a szárakat támadja meg, de a leveleket, hüvelyeket, levélhüvelyeket, levélkéket, levélnyakat és még a magokat is megfertőzheti. Kezdetben vörösesbarna elváltozások jelennek meg, majd a betegség előrehaladtával a pustulákban fekete teliospórák képződnek. A vörös téglatestű urediniospórák tömegei először a szárakon és a levélhüvelyeken alakulnak ki, és apró klorotikus foltok jelennek meg, amelyek lineáris vagy rombusz alakú pusztafoltokká fejlődnek, amelyek mérete megnőhet. Éréskor az urediniospórák termelése leáll, és egy fekete teliospóraréteg keletkezik, ami miatt a szárak a szezon végén feketének tűnnek. Súlyos esetekben a fertőzés meggyengíti a növény szárát, ami a növény elnyúlásához vezet.
Puccinia striiformis
A jellegzetes jellemzője a P. striiformis az egyenes oldalú sárga pustulák jelenléte. A TJey különböző hosszúságú, keskeny, hosszúkás csíkokban jelenik meg, gyakran klorózis és nekrózis kíséretében. A betegség érésével a pustulákon belül sárgás-narancsos spórák fejlődnek, a környező szövetek pedig barnává válnak és kiszáradnak, ami perzselt megjelenést eredményez.
FieldClimate modell
Puccinia rozsda modell
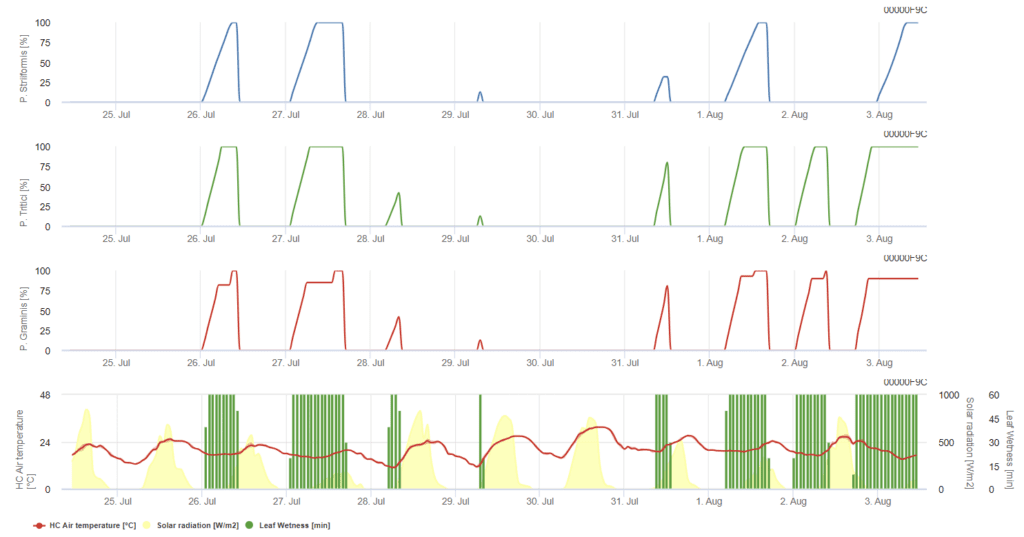
P. triticina, P. graminis, P. striiformis mindegyike három különböző színű grafikonon kerül bemutatásra.
Puccinia triticna fertőzési modell
A modell feltételezi, hogy a fertőzéshez 90 °C-os óránkénti összesített levélnedvességre van szükség 5 °C és 30 °C közötti léghőmérséklet-tartományban. A feltételek hasonlóak a következőkhöz P. graminis, de alacsonyabb, 5°C-os hőmérsékleti küszöbértékkel.
Az 100% fertőzés kimutatása esetén gyógyító növényvédelmi mérést kell fontolóra venni. Ha a kockázat 80%-nél van, és az időjárás-előrejelzés több levélnedves időszakot jósol, védekező levélkezelésre kerülhet sor.
A levelek nedvességtartalma 90°C-os felhalmozott óránkénti átlaghőmérséklet esetén:
- (ha T <= 22,5°C akkor ∑(Th) egyébként ∑ (22,5-(Th-22,5))
- 5°C < Temp. < 30°C
Puccinia graminis fertőzési modell
A fertőzés néhány órával a levél nedvesedése után, optimális hőmérsékleti viszonyok mellett következik be. A modell feltételezi, hogy a fertőzéshez 80°C-os felhalmozott óránkénti levélnedvességre van szükség a 10°C és 35°C közötti léghőmérséklet-tartományban. A betegség a valamivel magasabb hőmérsékletet részesíti előnyben, mint a P. triticina és a fertőzést napfénynek kell követnie.
Az 100% fertőzés kimutatásakor a körülmények kedvezőek voltak a gomba számára, ezért növényvédelmi intézkedéseket kell fontolóra venni.
A levélnedvesség 80°C-os felhalmozott óránkénti átlaghőmérséklethez, majd egy fényes időszak (150 W/m²) 30°C-os felhalmozott óránkénti átlaghőmérséklethez:
- (ha T <= 24°C, akkor ∑(Th), különben ∑ (Th-24))
- 10°C < Temp. < 35°C
Puccinia striiformis fertőzési modell
Puccinia striiformis a hűvös éghajlatú területek búzarozsdája, amelynek optimális hőmérséklete már 15 °C-tól kezdődik. A fertőzés néhány órás levélnedvesedés után, optimális hőmérsékleti viszonyok között következik be. A modell feltételezi, hogy a fertőzéshez 80°C-os felhalmozott óránkénti levélnedvességre van szükség 5°C és 20°C közötti léghőmérséklet-tartományban. Alacsony fényintenzitású időszakokban nem következik be fertőzés.
Mielőtt a grafikon eléri az 100% értéket, védekező alkalmazást lehet végezni, míg ezt követően gyógyító (szisztémás) védekezési stratégiákat kell fontolóra venni.
A levélnedvesség és a fény a 80°C-os felhalmozott óránkénti átlaghőmérsékletre:
- (ha T <= 15°C akkor ∑(Th) egyébként ∑ (Th-15))
- 5°C < Temp. < 20°C
Irodalom
- Bolton, M. D., Kolmer, J. A., & Garvin, D. F. (2008). A Puccinia triticina által okozott búzalevélrozsda. Molekuláris növénypatológia, 9(5), 563-575.
- Chen, X. M. (2005). A csíkrozsda [Puccinia striiformis f. sp. tritici] epidemiológiája és ellenőrzése búzán. Canadian journal of plant pathology, 27(3), 314-337.
- Figueroa, M., Hammond-Kosack, K. E., & Solomon, P. S. (2018). A búza betegségeinek áttekintése - szántóföldi szemlélet. Molekuláris növénypatológia, 19(6), 1523-1536.
- Guide, A. Leaf, Stem, and Stripe Rust Diseases of Wheat. Leonard, K. J., & Szabo, L. J. (2005). A Puccinia graminis által okozott kisméretű gabonafélék és fűfélék szárrozsdája. Molekuláris növénypatológia, 6(2), 99-111.
- Kolmer, J. (2013). A búza levélrozsdája: kórokozóbiológia, variáció és gazdaszervezeti rezisztencia. Erdők, 4(1), 70-84.
- Singh, R. P., Huerta-Espino, J., Roelfs, A. P., & Curtis, B. C. (2002). A búza rozsdásodik. Növekedés, 2(25), 35.
Ajánlott felszerelés
Ellenőrizze, hogy melyik érzékelőkészletre van szükség a növény potenciális betegségeinek megfigyeléséhez.
